Antimony (III, V) oxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ Sb 3 + / 5 + __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Antimony (III, V) oxide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | Sb 2 O 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 307.51 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
1050 ° C (decomposition) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−907.5 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Antimony (III, V) oxide , also antimony (III) antimonate (V) or diantimony tetroxide , is an antimony compound with the empirical formula Sb 2 O 4 . It belongs to the oxide class . Diantimony tetroxide is a white powder that is yellow when heated and is insoluble in water.
Occurrence
Antimony (III, V) oxide occurs naturally in the form of the mineral cervantite . Find places are among others in Romania , Bolivia and Spain .
Extraction and presentation
Antimony (III, V) oxide forms are available in several ways. Antimony (V) oxide converts to diantimony tetroxide at 800 ° C with the release of oxygen. However, the connection does not melt.
Diantimony tetroxide is also formed from antimony (III) oxide when it is heated to over 500 ° C in air.
properties
There are no tetravalent ions in antimony (III, V) oxide, rather it is a mixed oxide of antimony (III) and antimony (V) oxide. The frequently used name antimony (IV) oxide is therefore chemically incorrect.
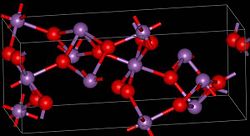
Two modifications , α- and β-antimony (III, V) oxide are known. At a temperature of 1130 ° C, the α- converts to the β-form. α-Antimony (III, V) oxide crystallizes in the orthorhombic crystal system with the lattice parameters a = 5.43 Å , b = 4.81 Å and c = 11.78 Å as well as four formula units per unit cell . The β-form is monoclinic with the lattice parameters a = 12.06 Å, b = 4.84 Å, c = 5.38 Å and β = 104.56 °, as well as four formula units per unit cell.
There are layers of SbO 5 octahedra that have a pentavalent antimony atom in the center. These are linked to one another via their corners. The layers are linked to one another via the trivalent antimony atoms.
Diantimony tetroxide is soluble in concentrated sulfuric acid and hydrochloric acid . In contrast, the compound does not dissolve in water or dilute acids and alkalis.
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 820.
- ↑ a b c J. Amador, E. Gutierrez Puebla, MA Monge, I. Rasines, C. Ruiz Valero: Diantimony Tetraoxides Revisited , in: Inorg. Chem. 1988 , 27 , 1367-1370, doi : 10.1021 / ic00281a011 .
- ↑ a b c Entry on antimony oxides. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ a b Strem Chemicals: Antimony (IV) oxide (99.99% -Sb) PURATREM , accessed on December 27, 2019
- ↑ M. Binnewies, E. Milke: Thermochemical Data of Elements and Compunds . 2nd Edition. Wiley-VCH, weinheim 2002, ISBN 3-527-30524-6 , pp. 829 .
- ^ Paul Ramdohr , Hugo Strunz : Textbook of Mineralogy . 16th edition. Ferdinand Enke Verlag 1978, ISBN 3-432-82986-8 (p. 543).
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 849.



