Barium selenate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
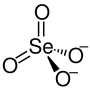
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Barium selenate | |||||||||||||||
| other names |
Barium selenate (VI) |
|||||||||||||||
| Molecular formula | BaSeO 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 280.28 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.75 g cm −3 |
|||||||||||||||
| Melting point |
> 350 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Barium selenate is an inorganic chemical compound of barium from the selenate group .
Extraction and presentation
Barium selenate can be obtained by reacting sodium selenate with barium chloride in an aqueous solution.
properties
Barium selenate is a white solid that is sparingly soluble in water. If heated above 425 ° C, the compound decomposes. Another barium selenate is also known with barium diselenate BaSe 2 O 7 . It has an orthorhombic crystal structure of the barite type with the space group Pnma (space group no. 62) (a = 8.993 Å , b = 5.675 Å, c = 7.349 Å).
use
Barium selenate was used as a “slow release” selenium source for forage plants for grazing animals and was intended to ensure the selenium supply of grazing animals. In Switzerland and the EU, direct use as a feed additive is prohibited. By reduction of Barium in the hydrogen stream can Bariumselenid be won.
Individual evidence
- ↑ a b c d e f Entry on barium selenate in the GESTIS substance database of the IFA , accessed on May 25, 2017(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Inorganic Compounds, pp. 4-50.
- ^ Karl A. Hofmann: Inorganic Chemistry . Springer-Verlag, 2013, ISBN 978-3-663-14240-9 , pp. 190 ( limited preview in Google Book Search).
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the indicated labeling it falls under the group entries for barium salts, with the exception of barium sulphate, salts of 1-azo-2-hydroxynaphthalenyl aryl sulphonic acid , and of salts specified elsewhere in this Annex and selenium compounds with the exception of cadmium sulphoselenide and those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 18, 2017. Manufacturers or distributors can use the expand harmonized classification and labeling .
- ^ A b Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 184 ( limited preview in Google Book search).
- ^ A. Andara, MA Salvado, Á. Fernández-González, S. García-Granda, M. Prieto: Crystal structure of barium selenate, BaSeO 4 . In: Journal of Crystallography - New Crystal Structures . 220, 2005, doi : 10.1524 / ncrs.2005.220.14.5 .
- ↑ lfl.bayern.de: AGGF conference proceedings Nutritional value and milk production potential of forage from grassland - Influence of the application of a calcium granulate containing selenium on the selenium concentration in pasture growth and the glutathione peroxidase activity of cattle of different breeds , H. Laser, M. Behrendts and B. Tönepöhl, accessed on June 15, 2016
- ↑ Entry on barium selenate at Vetpharm, accessed on June 15, 2016.
- ↑ europa.eu: IMPLEMENTING REGULATION (EU) 2015/446 OF THE COMMISSION of March 17, 2015 amending Regulation (EU) No. 37/2010 with regard to the substance "barium selenate" , accessed on June 15, 2016
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 949.




