Lithium amide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
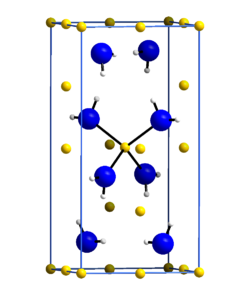
|
||||||||||||||||
| __ Li + __ N 3− __ H + | ||||||||||||||||
| Crystal system | ||||||||||||||||
| Space group |
I 4 (No. 82) |
|||||||||||||||
| Lattice parameters |
a = 504.309 pm |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Lithium amide | |||||||||||||||
| Ratio formula | LiNH 2 | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 22.96 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.178 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
373 ° C |
|||||||||||||||
| boiling point |
430 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lithium amide is a chemical compound of the lithium from the group of amides .
Extraction and presentation
Lithium amide is produced industrially by heating lithium or lithium hydride in a stream of ammonia . It also forms slowly when lithium is dissolved in liquid ammonia. As with other alkali and alkaline earth amides, this reaction is considerably accelerated by the presence of iron (II) chloride .
properties
Lithium amide is a colorless and odorless solid that decomposes in water.
The compound is generally very sensitive to hydrolysis, crystallizes tetragonally with space group I 4 (No. 82) and attacks glass weakly. When heated in a vacuum, it gives off ammonia above 300 ° C. However, quantitative degradation to lithium imide only takes place at 400 ° C and only above 750-800 ° C does it decompose with the release of nitrogen, hydrogen and ammonia.
use
Lithium amide is used in organic chemistry in Claisen condensations , in the alkylation of nitriles and ketones, and in the synthesis of ethynyl compounds and carbinols . It is still used as a reagent for the cross-coupling of aryl chlorides and amines .
Individual evidence
- ^ WIF David, MO Jones, DH Gregory, CM Jewell, SR Johnson, A. Walton, PP Edwards: A mechanism for non-stoichiometry in the lithium amide / lithium imide hydrogen storage reaction . In: Journal of the American Chemical Society , 2007 , 129 (6) , pp. 1594-1601 doi : 10.1021 / ja066016s
- ↑ a b c d e f g h Lithium amide data sheet (PDF) from Merck , accessed on March 27, 2013.
- ↑ a b Roger Blachnik (Ed.): Pocket book for chemists and physicists . Volume III: Elements, Inorganic Compounds and Materials, Minerals . founded by Jean d'Ans, Ellen Lax. 4th, revised and revised edition. Springer, Berlin 1998, ISBN 3-540-60035-3 , pp. 536 ( limited preview in Google Book search).
- ↑ a b Entry on lithium amide in the Hazardous Substances Data Bank , accessed on March 28, 2013.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 666.
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 448.
- ↑ Data sheet Lithium amide, powder, 95% from Sigma-Aldrich , accessed on March 28, 2013 ( PDF ).





