Barium permanganate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
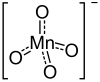
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Barium permanganate | |||||||||||||||
| other names |
Barium manganate (VII) |
|||||||||||||||
| Molecular formula | Ba (MnO 4 ) 2 | |||||||||||||||
| Brief description |
brown purple to black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 375.20 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.77 g cm −3 |
|||||||||||||||
| solubility |
625 g / l at 20 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Barium permanganate is an inorganic chemical compound of barium from the group of permanganates .
Extraction and presentation
Barium permanganate can be obtained by reacting barium manganate with carbon dioxide in water or with sulfuric acid.
Barium permanganate can also be made from silver permanganate and barium chloride . High purity barium can be obtained by reaction of potassium permanganate with aluminum sulfate are prepared, wherein the formed Aluminiumpermanganat with a stoichiometric amount of barium hydroxide is reacted.
properties
Barium permanganate is a brown-violet to black solid that is sparingly soluble in water. It is a strong oxidizing agent and decomposes to barium manganate at high temperatures above 280 ° C.
It is thermally stable up to 180 ° C and decomposes in two stages between 180–350 and 500–700 ° C. The decomposition takes place quickly from temperatures of 200–220 ° C, is exothermic and the activation energy is 113 kJ / mol. The decomposition can be described as follows.
Investigations have shown that decomposition begins slowly at 160 ° C and that this temperature continues to drop due to the action of UV light or X-rays (activation). Crystal defects and impurities play a role in the decomposition temperature and the decomposition mechanism. The compound does not occur as a hydrate, a trihydrate is known as a mixed crystal only in a matrix of barium perchlorate trihydrate.
Barium permanganate has an orthorhombic crystal structure with the space group Fddd (space group no. 70) (a = 14.749, b = 11.896, c = 7.414 Å , Z = 8). The average O-Mn distances are 1.74 and 1.61 Å.
use
By reacting barium permanganate with sulfates , permanganates of various metals can be produced.
Individual evidence
- ↑ a b Richard P. Pohanish: Sittig's Handbook of Toxic and Hazardous Chemicals and Carcinogens . William Andrew, 2011, ISBN 1-4377-7870-4 , pp. 323 ( limited preview in Google Book search).
- ↑ a b c d Georg Brauer: Handbook of preparative inorganic chemistry . 3., reworked. Edition. tape III . Enke, Stuttgart 1981, ISBN 3-432-87823-0 , pp. 1585 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Inorganic Compounds, pp. 4-50.
- ↑ Entry on barium permanganate in the GESTIS substance database of the IFA , accessed on July 10, 2016(JavaScript required) .
- ↑ a b c d Laszlo Kotai, Istvan Gacs, Istvan E. Sajo, Pradeep K. Sharma, Kalyan K. Banerji: ChemInform Abstract: Beliefs and Facts in Permanganate Chemistry - An Overview on the Synthesis and the Reactivity of Simple and Complex Permanganates. In: ChemInform. 42, 2011, S. no, doi : 10.1002 / chin.201113233 .
- ^ Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 895 ( limited preview in Google Book Search).
- ↑ EG Prout, PJ Herley: THE THERMAL DECOMPOSITION OF BARIUM PERMANGANATE. In: The Journal of Physical Chemistry. 65, 1961, p. 208, doi : 10.1021 / j100820a005 .







