Silver permanganate
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Ag + __ Mn 7+ __ O 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Silver permanganate | ||||||||||||||||||
| other names |
Silver manganate (VII) |
||||||||||||||||||
| Ratio formula | AgMnO 4 | ||||||||||||||||||
| Brief description |
black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 226.8 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
4.49 g cm −3 |
||||||||||||||||||
| Melting point |
160 ° C (decomposition) |
||||||||||||||||||
| solubility |
poorly soluble in water (silver permanganate 9 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Silver permanganate is an inorganic chemical compound of silver from the group of permanganates .
Extraction and presentation
Silver permanganate can be obtained by reacting a silver nitrate solution with potassium permanganate at 80 ° C.
properties
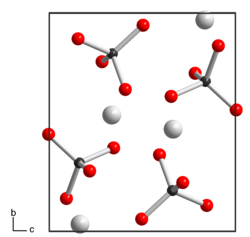
Silver permanganate is a light-sensitive purple to black solid with shiny metallic, needle-shaped crystals that is sparingly soluble in water. Decomposition begins when heated to over 160 ° C or when stored for a long time. It has a monoclinic crystal structure with the space group P 2 1 / n (space group no. 14, position 2) and the lattice parameters a = 567 pm, b = 827 pm, c = 713 pm, β = 92.5 °.
use
Silver permanganate is used in gas masks and as an antiseptic .
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1586.
- ↑ a b c d Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . Taylor & Francis US, 2011, ISBN 1-4398-1462-7 , pp. 369 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
