Gold (III) chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Gold (III) chloride | |||||||||||||||
| other names |
Gold trichloride |
|||||||||||||||
| Molecular formula | AuCl 3 (Au 2 Cl 6 ) | |||||||||||||||
| Brief description |
dark orange-red crystal needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 303.33 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.9 g cm −3 |
|||||||||||||||
| Melting point |
254 ° C (decomposition) |
|||||||||||||||
| solubility |
good in water (680 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
not yet determined |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−117.6 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Gold (III) chloride with the ratio formula AuCl 3 (and the empirical formula Au 2 Cl 6 ) is one of the most important gold compounds . Other gold chlorides are AuCl and AuCl 2 . However, AuCl 2 is not a gold (II) compound, but a mixed-valence Au (I) - / Au (III) compound.
Extraction and presentation
Gold (III) chloride is produced by passing chlorine gas over finely divided gold at 250 ° C.
properties
Physical Properties
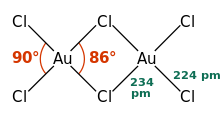
AuCl 3 is present both in the solid state and in the vapor phase as the dimer Au 2 Cl 6 . The same applies to the gold bromide AuBr 3 . In contrast to the likewise dimeric aluminum chloride , the gold (III) chloride dimers are arranged flat, the AuCl 4 unit is square-planar. In the case of (AlCl 3 ) 2 it has a tetrahedral structure and the bridging atoms are above and below the plane. The crystal structure is monoclinic with the space group P 2 1 / c (space group no. 14) and the lattice parameters a = 6.57 Å , b = 11.04 Å, c = 6.44 Å and β = 113.3 °. The Au-Cl bond has a strong covalent structure, which is due to the (comparatively) high electronegativity of gold and the high oxidation state.
Gold (III) chloride is very hygroscopic and readily soluble in water and ethanol.
Chemical properties
With +3 this is the most stable oxidation state of gold in compounds and complexes .
At temperatures above 250 ° C, AuCl 3 breaks down into AuCl and Cl 2 .
AuCl 3 is a Lewis acid and forms many complexes of the form M + AuCl 4 - (tetrachloroaurate). M can be, for example, potassium ( potassium tetrachloroaurate (III) ). The AuCl 4 - ion is not very stable in aqueous solution.
In hydrochloric acid to dissolve AuCl 3 with formation of chloroauric acid .
In aqueous solution, AuCl 3 reacts with alkali hydroxides ( e.g. sodium hydroxide ) to form Au (OH) 3 . When heated in air, this reacts to form gold (III) oxide Au 2 O 3 and further to metallic gold .
use
Gold (III) chloride is often used as the starting material for the production of other gold compounds and complexes. One example is the preparation of the cyanide complex KAu (CN) 4
Applications in organic chemistry :
Gold (III) salts , especially NaAuCl 4 (from AuCl 3 and sodium chloride ) can be used in organic synthesis as a catalyst in reactions with alkynes . There they serve as a non-toxic substitute for mercury (II) salts. An important example is the hydration of terminal alkynes to methyl ketones in high yield. Amines can also be made in a similar way.
Gold (III) chloride can be used as a mild catalyst for the alkylation of aromatic and heteroaromatic compounds. One example is the alkylation of 2-methylfuran with methyl vinyl ketone .
A phenol can be formed from furan derivatives and alkynes in a rearrangement with catalysis by gold (III) chloride .
literature
- The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, ISBN 978-0-911910-00-1 , p. 780.
Individual evidence
- ↑ a b c d Entry on gold (III) chloride in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c data sheet gold (III) chloride from AlfaAesar, accessed on January 18, 2010 ( PDF )(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-5.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1013.
- ^ ES Clark, DH Templeton, CH MacGillavry: The crystal structure of gold (III) chloride. In: Acta Crystallographica , 11, 1958, pp. 284-288, doi: 10.1107 / S0365110X58000694 .
- ↑ Y. Fukuda, K. Utimoto: Effective Transformation of Unactivated Alkynes into Ketones or Acetals by Means of Au (III) Catalyst . In: J. Org. Chem. , 1991 , 56 , pp. 3729-3731, doi: 10.1021 / jo00011a058 .
- ↑ G. Dyker: An Eldorado for Homogeneous Catalysis? In: Organic Synthesis Highlights V , H.-G. Schmalz, T. Wirth (eds.), Wiley-VCH, Weinheim 2003, pp. 48-55.
- ^ ASK Hashmi, TM Frost, JW Bats: Highly Selective Gold-Catalyzed Arene Synthesis. In: J. Am. Chem. Soc. , 2000 , 122 , pp. 11553-11554, doi: 10.1021 / ja005570d