Potassium carbide
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| General | ||||||||||
| Surname | Potassium carbide | |||||||||
| other names |
Dipotassium acetylide |
|||||||||
| Molecular formula | K 2 C 2 | |||||||||
| Brief description |
yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 102.22 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Potassium carbide (dipotassium dicarbide) is a chemical compound from the group of carbides . In addition to this compound, KC 8 , KC 16 and other potassium carbides are known.
Extraction and presentation
Potassium carbide can be produced in a single phase by reacting potassium dissolved in liquid ammonia with acetylene and then heating the hydrogen acetylide obtained in a high vacuum. The first synthesis of sodium carbide and potassium carbide was carried out by Moissan in the late 19th century. Depending on the temperature, KHC 2 can also arise.
The connection cannot be established by the reaction of potassium with carbon .
Potassium carbide can also be obtained by decomposing potassium methoxide at 300 ° C or by reacting potassium cyanide with magnesium .
properties
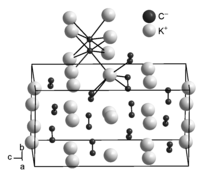
Potassium carbide is a yellow solid that ignites on contact with water. The compound crystallizes tetragonally in the space group I 4 1 / acd (space group no. 142) and can be interpreted as a distorted variant of the antifluorite structure. At temperatures above room temperature (420 K), in analogy to the alkaline earth metal acetylides, a reversible phase transformation (1st order) into a cubic high-temperature modification with the space group Fm 3 m (space group no. 225) occurs, which has an undistorted antifluorite structure corresponds to disordered C 2 2– dumbbells.
use
Potassium carbide can be used to make ethynyl steroids and 1,4-dicarbonyl compounds.
Individual evidence
- ^ A b c d e T. Y. Kosolapova: Carbides Properties, Production, and Applications . Springer Science & Business Media, 2012, ISBN 978-1-4684-8006-1 , p. 64 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b S. Hemmersbach, B. Zibrowius, U. Ruschewitz: Na 2 C 2 and K 2 C 2 : synthesis, crystal structure and spectroscopic properties. In: Journal of Inorganic and General Chemistry. 625, 1999, p. 1440, doi : 10.1002 / (SICI) 1521-3749 (199909) 625: 9 <1440 :: AID-ZAAC1440> 3.0.CO; 2-R .
- ↑ Chris Woodford: Potassium . Marshall Cavendish, 2003, ISBN 978-0-7614-1463-6 , pp. 11 ( limited preview in Google Book search).
- ↑ L. Bretherick: Bretherick's Handbook of Reactive Chemical Hazards . Elsevier, 2016, ISBN 978-1-4831-6250-8 , pp. 1996 ( limited preview in Google Book Search).
- ^ Encyclopedia of Reagents for Organic Synthesis . John Wiley & Sons, Ltd, 2001, ISBN 978-0-470-84289-8 , Dipotassium Acetylide, doi : 10.1002 / 047084289x.rd450 / abstract .