Platinum (IV) bromide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
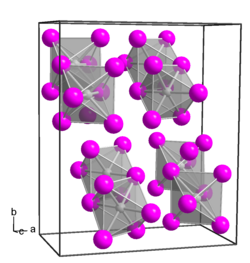
|
||||||||||||||||
| __ Pt 4+ __ Br - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Platinum (IV) bromide | |||||||||||||||
| other names |
Platinum tetrabromide |
|||||||||||||||
| Ratio formula | PtBr 4 | |||||||||||||||
| Brief description |
dark red solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 514.69 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
5.687 g cm −3 |
|||||||||||||||
| Melting point |
180 ° C (decomposition) |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Platinum (IV) bromide is an inorganic chemical compound of platinum from the group of bromides .
Extraction and presentation
Platinum (IV) bromide, by reaction of Platinbromwasserstoffsäure H 2 [PtBr 6 ] · 9H 2 O (formed by reaction of platinum with hydrobromic acid can be represented) with bromine are recovered.
It can also be obtained by reacting platinum with bromine, the reaction taking place very slowly.
properties
Platinum (IV) bromide is a dark red to black-violet solid that is soluble in water to form H 2 [PtBr 4 (OH) 2 ]. It is very easily soluble in ethanol and in ether and has an orthorhombic crystal structure with the space group Pbca (space group no. 61) and the lattice parameters a = 1199 pm, b = 1365 pm and c = 633 pm.
Individual evidence
- ↑ a b c d e f g data sheet Platinum (IV) bromide, 99.99% (metals basis), Pt 37.1% min from AlfaAesar, accessed on August 29, 2013 ( PDF )(JavaScript required) .
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer, 1998, ISBN 3-642-58842-5 , pp. 674 ( limited preview in Google Book search).
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1713.
- ^ JJ Zuckerman: Inorganic Reactions and Methods, The Formation of Bonds to Halogens . John Wiley & Sons, 2009, ISBN 0-470-14539-0 , pp. 174 ( limited preview in Google Book search).
