Actinium (III) fluoride
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ Ac 3+ __ F - | |||||||
| Crystal system | |||||||
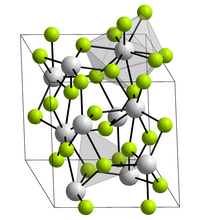
| Space group |
P 3 c 1 (No. 165) |
||||||
| Lattice parameters |
a = 741 pm |
||||||
| Coordination numbers |
Ac [9], F [3] |
||||||
| General | |||||||
| Surname | Actinium (III) fluoride | ||||||
| other names |
|
||||||
| Ratio formula | AcF 3 | ||||||
| Brief description |
white solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 284.02 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
7.88 g cm −3 |
||||||
| Hazard and safety information | |||||||
 Radioactive |
|||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Actinium (III) fluoride is a chemical compound of the elements actinium and fluorine .
presentation
Actinium (III) fluoride can be prepared either in solution or by solid reaction. In the first case, hydrofluoric acid is added to an Ac 3 solution at room temperature and the product is precipitated.
In the other case, actinium metal is treated with hydrogen fluoride at 700 ° C in a platinum apparatus.
properties
Physical Properties
Actinium (III) fluoride is a white solid. It crystallizes trigonally in the space group P 3 c 1 (space group no. 165) with the lattice parameters a = 741 pm and c = 755 pm and six formula units per unit cell .
Chemical properties
The conversion of actinium (III) fluoride with moist ammonia at 900–1000 ° C yields the oxyfluoride AcOF.
While lanthanum oxyfluoride is easily formed by heating lanthanum (III) fluoride in air at 800 ° C in about an hour, a similar treatment of actinium (III) fluoride does not yield AcOF and only results in a melt of the original substance.
safety instructions
Classifications according to the GHS regulation are not available because they only include chemical hazard, which plays a completely subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
Individual evidence
- ^ A b W. H. Zachariasen: Crystal Chemical Studies of the 5 f Series of Elements. XII. New Compounds Representing Known Structure Types. In: Acta Crystallographica . 2, 1949, pp. 388-390, doi : 10.1107 / S0365110X49001016 .
- ↑ Gerd Meyer, Lester R. Morss: Synthesis of lanthanide and actinide compounds. Springer, 1991, ISBN 0-7923-1018-7 , p. 71 ( limited preview in Google book search).
- ^ A b c Sherman Fried, French Hagemann, WH Zachariasen: The Preparation and Identification of Some Pure Actinium Compounds. In: J. Am. Chem. Soc. 72, 1950, pp. 771-775, doi : 10.1021 / ja01158a034 .
- ^ A b c Gerd Meyer, Lester R. Morss: Synthesis of lanthanide and actinide compounds. Springer, 1991, ISBN 0-7923-1018-7 , pp. 87-88 ( limited preview in Google book search).
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this substance has either not yet been classified or a reliable and citable source has not yet been found.

