Europium (II) hydride
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
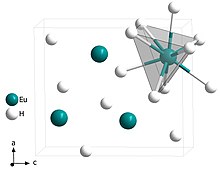
|
|||||||
| Crystal system |
orthorhombic |
||||||
| Space group |
Pnma (No. 62) |
||||||
| Lattice parameters |
|
||||||
| General | |||||||
| Surname | Europium (II) hydride | ||||||
| Ratio formula | EuH 2 | ||||||
| Brief description |
dark brown purple |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 153.98 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Europium (II) hydride is a dark brown violet inorganic chemical compound of europium from the group of hydrides .
Extraction and presentation
Europium (II) hydride is synthesized by hydrogenating europium metal at 600 K and 2 MPa hydrogen pressure.
properties
Europium (II) hydride crystallizes orthorhombically in the space group Pnma (space group no.62 ) with a = 624.5 (9) pm, b = 379.0 (6) pm and c = 720.7 (9) pm in the PbCl 2 structure, in which each europium atom is surrounded nine times by hydride anions, in the form of triple-capped trigonal prisms . This compound decomposes under air oxidation and is less than 18.3 K ferromagnetic . EuH 2 is used for the synthesis of other europium hydrides and for the production of europium (II) oxide .
Individual evidence
- ↑ a b R. Bischof, E. Kaldis, P. Wachter: Synthesis, crystallographic and physical properties of europium dihydride . In: Journal of the Less-Common Metals . tape 111 , no. 1-2 , September 1985, pp. 139-144 , doi : 10.1016 / 0022-5088 (85) 90179-1 .
- ↑ a b c N. Kunkel, H. Kohlmann, A. Sayede, M. Springborg: Alkaline-Earth Metal Hydrides as Novel Host Lattices for Eu II Luminescence . In: Inorganic Chemistry . tape 50 , no. June 13 , 2011, p. 5873-5875 , doi : 10.1021 / ic200801x .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Daniel Rudolph: Structural and spectroscopic investigations on mixed anionic hydrides and oxide halides of the rare earth metals . Dr. Hut, Munich 2018, ISBN 978-3-8439-3653-8 .
- ↑ a b J. M., Haschke, MR Clark: Phase equilibria and crystal growth of alkaline earth and lanthanide dihydrides . In: High Temperature Science . tape 7 , no. 2 , 1975, p. 152-158 .
- ^ H. Kohlmann, K. Yvon: The crystal structures of EuH 2 and EuLiH 3 by neutron powder diffraction . In: Journal of Alloys and Compounds . tape 299 , no. 1-2 , March 2000, pp. L16-L20 , doi : 10.1016 / S0925-8388 (99) 00818-X .
- ↑ R. Bishop, E. Kaldis, P. Wachter: EUH 2 : A new ferro-magnetic semiconductor . In: Journal of Magnetism and Magnetic Materials . tape 31-34 , February 1983, pp. 255-256 , doi : 10.1016 / 0304-8853 (83) 90239-1 .
