Chromium (III) sulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
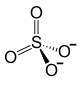
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Chromium (III) sulfate | ||||||||||||||||||
| other names |
Chromium sulfate |
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.01 g cm −3 (anhydrate at 20 ° C) |
||||||||||||||||||
| Melting point |
90 ° C |
||||||||||||||||||
| solubility |
bad in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chromium (III) sulfate (Cr 2 (SO 4 ) 3 ) is a salt of sulfuric acid .
presentation
Chromium (III) sulfate can be represented by the reduction of dichromates :
Chromium (III) sulfate can be produced by reacting potassium dichromate , sulfuric acid and hydrogen sulfide . Potassium sulphate and water are produced as by-products :
Alternatively, for example, ethanol can also be used instead of hydrogen sulfide, whereby - in addition to chromium (III) sulfate - potassium sulfate, acetic acid and water are formed:
However, chromium (III) sulfate containing water of crystallization is obtained via these reactions. The anhydrate can be produced by removing the water of crystallization at 280 ° C in a stream of CO 2 .
properties
Anhydrous chromium (III) sulfate forms red-brown, hexagonal crystals; with water of crystallization as the pentahydrate (Cr 2 (SO 4 ) 3 · 5 H 2 O) it is dark green. The dodecahydrate and octadecahydrate, on the other hand, are lavender to violet.
Absolutely anhydrous chromium (III) sulfate is insoluble in water, whereas the forms containing water of crystallization are water-soluble with the formation of blue to violet-colored aqua complexes , which change to green-colored sulfato complexes when heated.
The pentahydrate has a triclinic crystal structure, isotypic to that of copper sulfate pentahydrate.
use
Chromium (III) sulphate is used as a textile stain or for tanning leather. It is also used as a raw material for other chromium compounds.
Individual evidence
- ↑ a b SLAC: PHYSICAL CONSTANTS OF INORGANIC COMPOUNDS (PDF; 391 kB).
- ↑ a b c Entry on chromium (III) sulfate. In: Römpp Online . Georg Thieme Verlag, accessed on June 20, 2014.
- ↑ a b c entry to chromium (III) sulphate in the GESTIS database of IFA , retrieved on September 4, 2016(JavaScript required) .
- ↑ jtbaker.com: Chromium Sulfate .
- ↑ a b Datasheet Chromium (III) sulfate hydrate, Reagent Grade at AlfaAesar, accessed on December 7, 2019 ( PDF )(JavaScript required) .
- ↑ a b Gmelin's Handbook of Inorganic Chemistry - No. 52 Chromium, The compounds (without complex bonds with neutral ligands) , 8th edition Berlin, 1962.
- ↑ Jander-Blasius: Textbook of analytical and preparative inorganic chemistry , 5th edition, S. Hirzel, Stuttgart-Leipzig 1965, p. 225
- ↑ Georg Brauer (ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1509.

![{\ mathrm {\ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)






