Europium (II) silicate
| Crystal structure | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Europium (II) silicate | ||||||||||||
| Ratio formula | Eu 2 [SiO 4 ] | ||||||||||||
| Brief description |
lemon yellow solid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 396.01 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
6.734 g cm −3 |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Europium (II) silicate (Eu 2 [SiO 4 ]) is a chemical compound and is one of the ortho silicates of europium . In addition, the more highly linked silicate Eu[SiO 3 ] and the europium (III) -oxido- ortho -silicate Eu 2 O [SiO 4 ] mentioned.
Extraction and presentation
The compound can be produced by reducing europium (III) oxide with elemental europium and silicon dioxide at 1100 ° C.
Another synthesis method is the synthesis from europium (III) oxide and silicon dioxide in a hydrogen atmosphere.
properties
Europium (II) silicate is a lemon yellow solid that is resistant to air up to 250 ° C and oxidizes to Eu 2 O [SiO 4 ].
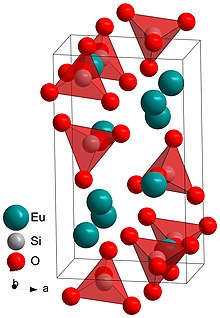
At room temperature, europium (II) silicate crystallizes in the larnite structure (Ca 2 [SiO 4 ]), in the monoclinic space group P 2 1 / n (space group no. 14, position 2) with four formula units per unit cell . Above 179 ° C, europium (II) silicate changes to a high-temperature modification and crystallizes there in an incommensurably modulated structure, i.e. a distorted variant of the orthorhombic space group Pnma (No. 62) . The atoms are periodically deflected from their lattice positions, but in no simple relation to the crystal lattice . Because of the isolated silicate units, it is an island silicate .
The compound glows intensely lemon yellow through luminescence under UV light and changes the color to orange above the phase transition .
Individual evidence
- ↑ a b c d e f g C. Funk, J. Köhler, I. Lazar, D. Kajewski, K. Roleder, J. Nuss, A. Bussmann-Holder, H. Bamberger, J. van Slageren, D. Enseling , T. Jüstel, T. Schleid, Old and New Insights into Structure and Properties of Eu 2 [SiO 4 ] , Cryst. Growth Des . 2018 , 18 , 6316-6325. doi: 10.1021 / acs.cgd.8b01265
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ H. Jacobsen, G. Meyer, W. Schipper, G. Blasse, Synthesis, structures and luminescence of two new Europium (II) Silicate ‐ Chlorides, Eu 2 SiO 3 Cl 2 and Eu 5 SiO 4 Cl 6 , Z. Anorg . General Chem. 1994 , 620 , 451-456, doi : 10.1002 / zaac.19946200308 .
- ^ ME Bohem, T. Schleid: The Luminescence Properties of Eu 2 O [SiO 4 ] and Eu 4,667 O [SiO 4 ] 3 In: Z. Anorg. General Chem. 2016 , 642 , 1058.
- ↑ J. Felsche, E. Kaldis, Thermal Oxidation of Eu 2 SiO 4 - a Topotactic Solid State Reaction . J. Solid State Chem. 1972 , 5 , 49-56. doi: 10.1016 / 0022-4596 (72) 90008-4
![{\ displaystyle {\ ce {2 Eu2O3 + 2 Eu + 3 SiO2 -> 3 Eu2 [SiO4]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3cee2af5a9fea2492f95c48a5bae641ba67306b3)