Calcium disilicide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
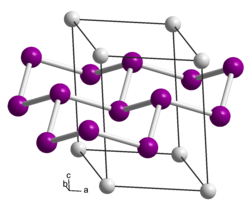
|
||||||||||||||||
| __ Ca 2+ __ Si - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Calcium disilicide | |||||||||||||||
| other names |
Calcium silicide (ambiguous) |
|||||||||||||||
| Ratio formula | CaSi 2 | |||||||||||||||
| Brief description |
gray solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 96.25 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.47 g cm −3 |
|||||||||||||||
| Melting point |
1040 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Calcium disilicide is an inorganic chemical compound of calcium with silicon and one of the well-known calcium silicides . It's a Zintl phase .
Extraction and presentation
Calcium disilicide can be obtained by reacting calcium or calcium hydride with silicon in a hydrogen atmosphere at about 1000 ° C,
be obtained by reacting silicon dioxide with calcium or by reducing a mixture of calcium oxide and silicon dioxide with carbon .
It can also be obtained by reducing silicon dioxide with coke in the presence of calcium carbide .
properties
Calcium disilicide is a gray solid. The connection discovered by Friedrich Wöhler in 1863 was examined by X-ray analysis by J. Böhm and O. Hassel in 1927. Then it crystallizes trigonal / rhombohedral in the space group R 3 m (space group no. 166) with the trigonal axes a = 3.86 Å and c = 30.6 Å. The structure can be described as a corrugated six-ring lattice made of silicon layers, between which the calcium atoms lie. A check has shown that a different calcium disilicide phase arises from pure calcium and pure silicon. According to this, the pure calcium disilicide has the same symmetry, but only about half the identity period in the c-direction. A polytype in the space group P 3 m 1 (No. 164) and the lattice parameters a = 3.85 Å and c = 5.20 Å is also known. There is also a tetragonal high temperature variant or high pressure variant with the space group I 4 1 / amd (No. 141) of the α- thorium disilicide type known. The conversion to the high-temperature form takes place between 600 and 1000 ° C, the reverse conversion when cooling below 530 ° C. A further phase change to the aluminum diboride type takes place above 16 GPa .
Calcium disilicide reacts with hydrogen chloride in ethanol to form polysilanes .
use
Calcium silicide is used in the manufacture of pharmaceuticals and in deoxidizers and desulfurizers , which can be used in the manufacture of many types of stainless steel . It is also used as a vaccine and additive in cast iron . It can also be used for the production of siloxenes , which was already discovered by Wöhler.
Individual evidence
- ↑ a b c d e data sheet Calcium silicide, technical at Sigma-Aldrich , accessed on July 6, 2017 ( PDF ).
- ↑ a b K. H. Janzon, Herbert Schäfer, Armin Weiss: Notes: On the structure of the CaSi2 phase. In: Zeitschrift für Naturforschung B. 23, 1968, doi : 10.1515 / znb-1968-1128 .
- ↑ a b c d data sheet Calcium silicide, tech. Ca ≈30%, may contain up to 5% Fe at AlfaAesar, accessed on July 6, 2017 ( PDF )(JavaScript required) .
- ^ A b William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 56 ( limited preview in Google Book search).
- ↑ Michelle JS Spencer, Tetsuya Morishita: Silicene Structure, Properties and Applications . Springer, 2016, ISBN 978-3-319-28344-9 , pp. 87 ( limited preview in Google Book search).
- ↑ Jane E. Macintyre: Dictionary of Inorganic Compounds . CRC Press, 1992, ISBN 978-0-412-30120-9 , pp. 2780 ( limited preview in Google Book search).
- ↑ Norbert Auner, Wolfgang A. Herrmann, Uwe Klingebiel: Synthetic Methods of Organometallic and Inorganic Chemistry, Volume 2, 1996 Volume 2: Groups 1,2, 13 and 14 . Georg Thieme Verlag, 2014, ISBN 3-13-179171-3 , p. 59 ( limited preview in Google Book search).
- ^ A b Egon Wiberg, Nils Wiberg: Inorganic Chemistry . Academic Press, 2001, ISBN 978-0-12-352651-9 , pp. 823 ( limited preview in Google Book search).
- ↑ J. Böhm, O. Hassel: The crystal structure of the calcium silicide CaSi2. In: Journal of Inorganic and General Chemistry. 160, 1927, p. 152, doi : 10.1002 / zaac.19271600115 .
- ^ Sarah M. Castillo, Zhongjia Tang, Alexander P. Litvinchuk, Arnold M. Guloy: Lattice Dynamics of the Rhombohedral Polymorphs of CaSi2. In: Inorganic Chemistry. 55, 2016, p. 10203, doi : 10.1021 / acs.inorgchem.6b01399 .
- ^ S. Fahy, DR Hamann: Electronic and structural properties of CaSi 2 . In: Physical Review, Series 3. B - Condensed Matter , 41, 1990, pp. 7587-7592, doi: 10.1103 / PhysRevB.41.7587 .
- ↑ R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 358 ( limited preview in Google Book Search).
- ^ Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 376 ( limited preview in Google Book search).
- ^ E. Yu Tonkov: Compounds and Alloys Under High Pressure A Handbook . CRC Press, 1998, ISBN 978-90-5699-047-3 , pp. 100 ( limited preview in Google Book search).
- ↑ P. Bordet, M. Affronte, S. Sanfilippo, M. Núñez-Regueiro, O. Laborde, GL Olcese, A. Palenzona, S. LeFloch, D. Levy, M. Hanfland: Structural phase transitions in under high pressure. In: Physical Review B. 62, 2000, p. 11392, doi : 10.1103 / PhysRevB.62.11392 .
- ^ JJ Zuckerman, AP Hagen: Inorganic Reactions and Methods, The Formation of the Bond to Hydrogen . John Wiley & Sons, 2009, ISBN 0-470-14537-4 , pp. 77 ( limited preview in Google Book search).
- ^ Ulrich Schubert: Silicon Chemistry . Springer Science & Business Media, 2012, ISBN 978-3-7091-6357-3 , p. 69 ( limited preview in Google Book search).



