Calcium permanganate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
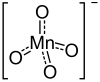
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Calcium permanganate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | Ca (MnO 4 ) 2 | |||||||||||||||
| Brief description |
purple solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 277.95 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.4 g cm −3 |
|||||||||||||||
| Melting point |
140 ° C (decomposition) |
|||||||||||||||
| solubility |
easily soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
0.5 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Calcium permanganate is a chemical compound made up of calcium , oxygen and manganese . It is the calcium salt of the permanganic acid HMnO 4, which is unknown in the free state . Which is exclusively for the intense color of the salt permanganate - anion responsible.
Extraction and presentation
Calcium permanganate can be obtained by electrolysis of solutions of alkali manganates and chlorinated lime or, as with magnesium permanganate, from calcium chloride and silver permanganate .
Chemical properties
Calcium permanganate Ca (MnO 4 ) 2 , like potassium permanganate, is a strong oxidizing agent .
use
Calcium permanganate is used:
- in textile production
- for bleaching paper
- for sterilizing water and during dental treatments
- as a catalyst in rocket fuels
- in coatings / fluxes of welding electrodes
- generally as an antiseptic, disinfectant and deodorant compound
Individual evidence
- ↑ a b c d e Entry on calcium permanganate in the GESTIS substance database of the IFA , accessed on February 27, 2017 (JavaScript required)
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ HazMap: Calcium permanganate
