Magnesium permanganate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
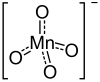
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Magnesium permanganate | |||||||||||||||
| Molecular formula | Mg (MnO 4 ) 2 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 262.18 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.18 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
100-113 ° C |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Magnesium permanganate is an inorganic chemical compound of magnesium from the group of permanganates .
Extraction and presentation
Magnesium permanganate hexahydrate was produced by E. Mitserlich and H. Aschoff by reacting silver permanganate with magnesium chloride or barium permanganate with magnesium sulfate . Starting from silver permanganate and magnesium chloride, slow crystallization and drying leads to the tetrahydrate. The anhydrate can be obtained by thermal dehydration of the hexahydrate.
properties
Magnesium permanganate hexahydrate is a blue black solid. It decomposes from 130 ° C with evolution of oxygen in an autocatalytic decomposition process. The tetrahydrate decomposes above 150 ° C. The crystals are practically insoluble in tri- and carbon tetrachloride , benzene , toluene , nitrobenzene ether , ligroin and carbon disulfide , but soluble in pyridine and glacial acetic acid. It dissolves in water and completely dissociates in dilute solutions. It oxidizes a number of organic compounds and reacts instantly (with fire in some cases) with common solvents such as THF, ethanol , methanol , t-butanol, acetone, and acetic acid.
use
Magnesium permanganate is used in various branches of industry and technology, e.g. B. as a wood impregnation agent, as an additive in tobacco filters, as a catalyst in the air oxidation of toluene to benzoic acid and in proteome research .
Individual evidence
- ↑ a b c d e data sheet Magnesium permanganate hydrate from Sigma-Aldrich , accessed on January 10, 2017 ( PDF ).
- ↑ a b c d Laszlo Kotai, Istvan Gacs, Istvan E. Sajo, Pradeep K. Sharma, Kalyan K. Banerji: ChemInform Abstract: Beliefs and Facts in Permanganate Chemistry - An Overview on the Synthesis and the Reactivity of Simple and Complex Permanganates. In: ChemInform. 42, 2011, S. no, doi : 10.1002 / chin.201113233 .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 72 ( limited preview in Google Book search).
- ^ Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 875 ( limited preview in Google Book search).



