Lithium sulfate
| Crystal structure | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
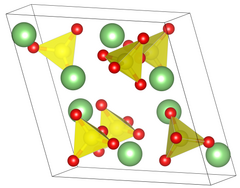
| __ Li + __ S 6+ __ O 2− | |||||||||||||
| Crystal system |
monoclinic |
||||||||||||
| Space group |
P 2 1 / a (No. 14, position 3) |
||||||||||||
| Lattice parameters |
a = 8.239 Å, b = 4.954 Å, c = 8.474 Å, β = 107.98 ° |
||||||||||||
| General | |||||||||||||
| Surname | Lithium sulfate | ||||||||||||
| other names |
Dilithium sulfate |
||||||||||||
| Ratio formula | Li 2 SO 4 | ||||||||||||
| Brief description |
White dust |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 109.94 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
2.22 g cm −3 |
||||||||||||
| Melting point |
845 ° C |
||||||||||||
| solubility |
good in water (342 g l −1 at 25 ° C) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Lithium sulfate is a chemical compound of lithium from the group of sulfates .
Extraction and presentation
Lithium sulfate is made by reacting lithium carbonate with sulfuric acid .
properties
Lithium sulfate also occurs as a monohydrate , which converts to anhydrate at 130 ° C. The monohydrate crystallizes in the monoclinic crystal system in the space group P 2 1 (space group no. 4) . The anhydrate also crystallizes in the monoclinic crystal system, but in the space group space group P 2 1 / c (space group no. 14) and 4 formula units in the unit cell .
use
Lithium sulfate is used in lithium therapy and in quick-setting cement . Lithium sulfate single crystals are also used in piezoelectrics , acoustics , for ultrasonic transmitters and for deflecting light.
Individual evidence
- ^ AG Nord: Crystal structure of β-Li 2 SO 4 . In: Acta Crystallographica Section B . tape 32 , 1976, pp. 982-983 , doi : 10.1107 / S0567740876004433 .
- ↑ a b c Entry on lithium sulfate in the GESTIS material database of the IFA , accessed on December 7, 2019(JavaScript required) .
- ↑ a b c d data sheet Lithium sulfate from Sigma-Aldrich , accessed on September 3, 2017 ( PDF ).
- ↑ a b Ulrich Wietelmann, Richard J. Bauer: lithium and lithium compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim 2005, doi : 10.1002 / 14356007.a15_393 .
- ^ Entry on Lithium sulfate in the ChemSpider database of the Royal Society of Chemistry , accessed on January 22, 2014.
- ↑ L. Bayarjargal, L. Bohatý: Nonlinear optical properties of lithium sulfate monohydrate, Li 2 SO 4 .H 2 O . In: Acta Crystallographica Section A: Foundations of Crystallography . tape 61 , A1, 2005, pp. 393-394 , doi : 10.1107 / S0108767305083339 .
- ^ YN Zhuravlev, LV Zhuravleva, OV Golovko: Chemical bond in alkali metal sulfates . In: Journal of Structural Chemistry . tape 48 , no. 5 , 2007, p. 789-795 , doi : 10.1007 / s10947-007-0120-y .
- ↑ Entry on lithium sulfate. In: Römpp Online . Georg Thieme Verlag, accessed on July 15, 2014.

