Sodium chromate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
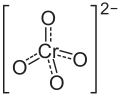
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sodium chromate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | Na 2 CrO 4 | ||||||||||||||||||
| Brief description |
odorless, translucent yellow crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 161.97 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.73 g cm −3 (18 ° C) |
||||||||||||||||||
| Melting point |
792 ° C |
||||||||||||||||||
| boiling point |
thermal decomposition |
||||||||||||||||||
| solubility |
good in water (530 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
of particular concern : carcinogenic, mutagenic, toxic for reproduction ( CMR ); subject to approval |
||||||||||||||||||
| MAK |
Switzerland: 5 μg m −3 (calculated as chromium) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sodium chromate is a chemical compound of the elements sodium , chromium and oxygen or the sodium salt of chromic acid .
Extraction and manufacture
It arises as an intermediate product in the extraction of chromium from the mineral chromite .
properties
Sodium chromate has a strong oxidizing effect.
use
The sodium salt of chromic acid is used in the laboratory as a strong oxidizing agent , as a marking substance in biological research, as an anti-corrosion agent in refrigerators and as a wood preservative .
safety instructions
Like all chromates , sodium chromate is carcinogenic, teratogenic and can lead to allergies . The substance is toxic if swallowed and very toxic if inhaled.
Individual evidence
- ↑ a b Sodium chromate data sheet at AlfaAesar, accessed on February 3, 2010 ( PDF )(JavaScript required) .
- ↑ a b c d e f Entry on sodium chromate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on sodium chromate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 14, 2014.
- ↑ Entry in the register of substances subject to authorization of the European Chemicals Agency , accessed on July 14, 2014.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for chromium (VI) compounds ), accessed on October 7, 2019.
- ↑ Entry on chrome at seilnacht.com, accessed on January 27, 2018.
- ^ I. Lauermann, H. Hecker, E. Kirchner: Creation of blood volume reference values for erythrocyte-marking indicator dilution methods on the basis of literature data. In: Transfusion Medicine and Hemotherapy. 26, 1999, p. 360, doi : 10.1159 / 000053520 .
- ↑ PAN Pesticides Database - California Pesticide Use: Sodium chromate - Pesticide use statistics for 2005 ( Memento of September 28, 2007 in the Internet Archive ), accessed on January 27, 2018.




