Antimony (III) sulfate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
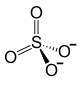
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Antimony (III) sulfate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | Sb 2 (SO 4 ) 3 | |||||||||||||||
| Brief description |
Colorless, hygroscopic, needle-shaped crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 531.71 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.62 g cm −3 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−2402.5 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sulfate antimony (III) is the antimony - salt of sulfuric acid .
Extraction and presentation
Antimony (III) sulfate can be produced by reacting antimony (III) oxide with hot, concentrated sulfuric acid.
Antimony (III) oxide and sulfuric acid react to form antimony (III) sulphate and water
Reactions
The antimony (III) oxide can be re-produced from (basic) antimony (III) sulfate by boiling it with strongly diluted sodium carbonate solution:
Antimony (III) sulfate reacts with sodium carbonate and water to form antimony (III) oxide, sodium sulfate and carbonic acid
safety instructions
Antimony (III) sulfate, like many other antimony compounds, should be classified as carcinogenic (Category 2) and germ cell mutagenic (Category 3B) according to the recommendation of the MAK Commission .
proof
The antimony cations of the antimony (III) sulfate can be detected with the Marsh sample .
The sulfate anions can be detected by means of the sulfate detection with an aqueous solution of barium chloride .
Individual evidence
- ↑ a b c Georg Brauer: Handbook of preparative inorganic chemistry. 1963.
- ↑ a b c d entry to antimony (III) sulfate in the GESTIS database of IFA , retrieved on February 1, 2016(JavaScript required) .
- ↑ M. Binnewies, E. Milke: Thermochemical Data of Elements and Compunds . 2nd Edition. Wiley-VCH, Weinheim 2002, ISBN 3-527-30524-6 , pp. 782 .
- ^ Concise dictionary of chemistry . Retrieved January 23, 2011.
literature
- Georg Brauer: Handbook of preparative inorganic chemistry. 1963.

![{\ mathrm {\ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)



