Tellurium trioxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
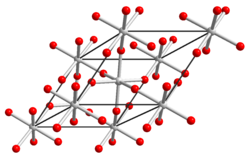
|
|||||||||||||||||||
| __ Te 6+ __ O 2− | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tellurium trioxide | ||||||||||||||||||
| other names |
Tellurium (VI) oxide |
||||||||||||||||||
| Ratio formula | TeO 3 | ||||||||||||||||||
| Brief description |
whitish to yellow-orange solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 175.60 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
5.07 g cm −3 |
||||||||||||||||||
| Melting point |
from 400 ° C decomposition to TeO 2 and oxygen |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tellurium trioxide is a chemical compound and the anhydride of orthotelluric acid H 6 TeO 6 .
properties
It is a yellow, trigonal / rhombohedral crystallizing solid and exists in two modifications , the X-ray amorphous yellow-orange α-form and the gray microcrystalline β-form. This crystallizes trigonal , space group R 3 c (space group no. 167) with the lattice parameters a = 5.195 Å and α = 56.38 °.
Extraction and presentation
The yellow-orange form arises when the orthotelluric acid is dehydrated at approx. 300-320 ° C. The yellow color comes about through the transfer of electrons from the oxygen to the tellurium (“ charge transfer ”). The gray β-form arises from the orthotelluric acid in the sealed tube at 320 ° C in the presence of conc. Sulfuric acid . The β form is essentially less reactive. This can be seen e.g. B. in the fact that it is not soluble in water, acids and even in hot alkalis. Both forms of tellurium trioxide decompose above approx. 400 ° C into tellurium (IV) oxide and oxygen.
Individual evidence
- ↑ a b web element .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Doctoral thesis RWTH Aachen .
- ↑ MAK Ahmed, H. Fjellvåg, A. Kjekshus: Synthesis, structure and thermal stability of tellurium oxides and oxide sulfate formed from reactions in refluxing sulfuric acid . In: Journal of the Chemical Society, Dalton Transactions , 2000, pp. 4542-4549, doi : 10.1039 / B005688J .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 .
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry , 2nd ed., Vol. 1, Academic Press 1963, pp. 450-451.