Tin (IV) bromide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Wedges to clarify the spatial structure | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tin (IV) bromide | ||||||||||||||||||
| other names |
Tin tetrabromide |
||||||||||||||||||
| Molecular formula | SnBr 4 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 438.33 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
3.34 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
31 ° C |
||||||||||||||||||
| boiling point |
202 ° C |
||||||||||||||||||
| solubility |
easily soluble in water |
||||||||||||||||||
| Refractive index |
1.6628 (31 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Tin (IV) bromide is an inorganic chemical compound of tin from the group of bromides .
Extraction and presentation
Tin (IV) bromide tetrahydrate can be obtained by reacting tin (IV) oxide with hydrobromic acid. The anhydrate can be obtained by reacting the elements.
properties
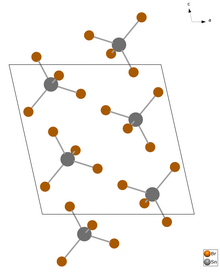
Tin (IV) bromide is a colorless solid that smokes in air and is easily soluble in water. It has a monoclinic crystal structure with the space group P 2 1 / c (space group no. 14) , a = 1037.1 pm, b = 700.6 pm, c = 1047.0 pm, β = 102.56 °, Z = 4.
use
Tin (IV) bromide as the starting material for tin (IV) oxide by laser-assisted chemical vapor deposition (engl. Laser-assisted CVD ) were used and thereby has a higher efficiency than other starting materials such as dibutyltin diacetate . It is also used to separate minerals.
Individual evidence
- ↑ a b c d e f g h data sheet Tin (IV) bromide, 99.999% trace metals basis from Sigma-Aldrich , accessed on July 23, 2013 ( PDF ).
- ^ A b c Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . Taylor & Francis, 2011, ISBN 1-4398-1462-7 , pp. 483 ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Liquids, pp. 4-140.
- ↑ Anil Kumar De: A Text Book of Inorganic Chemistry . New Age International, 2007, ISBN 81-224-1384-6 , pp. 379 ( limited preview in Google Book search).
- ↑ Roger Blachnik (Ed.): Paperback for chemists and physicists . Volume III: Elements, Inorganic Compounds and Materials, Minerals . founded by Jean d'Ans, Ellen Lax. 4th, revised and revised edition. Springer, Berlin 1998, ISBN 3-540-60035-3 , pp. 1389 ( limited preview in Google Book Search).
- ↑ H. Reuter, R. Pawlak: Zinnhalogenverbindungen. II. The molecular and crystal structures of tin (IV) bromide and iodide. In: Journal of Crystallography. 216, 2001, pp. 34-38, doi : 10.1524 / zkri.216.1.34.18992 .