Tin (II) iodide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| __ Sn 2+ __ I - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Tin (II) iodide | ||||||||||||||||||
| other names |
Tin diiodide |
||||||||||||||||||
| Ratio formula | SnI 2 | ||||||||||||||||||
| Brief description |
yellow to red solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 372.52 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
5.28 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
320 ° C |
||||||||||||||||||
| boiling point |
714 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Tin (II) iodide is an inorganic chemical compound of tin from the group of iodides .
Extraction and presentation
Tin (II) iodide can be obtained by reacting tin with iodine at more than 600 ° C, whereby tin (IV) iodide is first formed, which reacts with excess tin to form tin (II) iodide.
It can also be represented by reacting a tin (II) chloride solution with potassium iodide :
properties
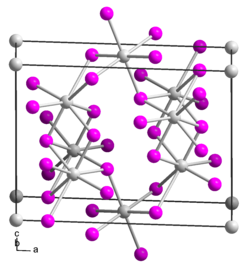
Tin (II) iodide is a yellow to red solid that is sparingly soluble in water. It has a monoclinic crystal structure with the space group C 2 / m (space group no. 12) , the lattice parameters a = 1417 pm, b = 453.5 pm, c = 1087 pm, 92 ° and six formula units per unit cell . It forms halogen complexes with halides.
Individual evidence
- ↑ Data sheet Tin (II) iodide, ultra dry, 99.999% (metals basis) from AlfaAesar, accessed on July 23, 2013 ( PDF )(JavaScript required) .
- ↑ a b c d e f g h i data sheet Tin (II) iodide, powder, 99.999% trace metals basis from Sigma-Aldrich , accessed on July 23, 2013 ( PDF ).
- ^ A b c Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists . Springer DE, 1997, ISBN 3-540-60035-3 , pp. 740 ( limited preview in Google Book search).
- ↑ Andrea Krüger: Structural chemical investigations of new binary and ternary ... Herbert Utz Verlag, 1999, ISBN 3-89675-472-6 , p. 25 ( limited preview in Google Book search).
- ↑ Anil Kumar De: A Text Book of Inorganic Chemistry . New Age International, 2007, ISBN 81-224-1384-6 , pp. 377 ( limited preview in Google Book search).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 965.



