Magnesium phosphide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
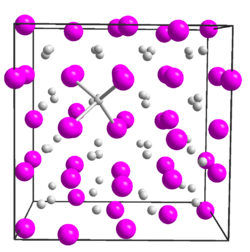
| __ Mg 2+ __ P 3− | ||||||||||||||||
| Crystal system |
cubic |
|||||||||||||||
| Space group |
Ia 3 (No. 206) |
|||||||||||||||
| Lattice parameters |
a = 12.01 Å |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Magnesium phosphide | |||||||||||||||
| other names |
Trimagnesium diphosphide |
|||||||||||||||
| Ratio formula | Mg 3 P 2 | |||||||||||||||
| Brief description |
gray-green pellets with a garlic-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 134.86 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.16 g cm −3 |
|||||||||||||||
| Melting point |
> 750 ° C |
|||||||||||||||
| solubility |
decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Magnesium phosphide is a compound from the group of phosphides . It is a gray-green solid with the smell of monophosphine , which is caused by the reaction with the humidity .
Manufacturing
Magnesium phosphide can be produced by adding liquid phosphorus to heated magnesium under an inert gas atmosphere.
properties
Magnesium phosphide can be mixed with various substances such as B. halogens , oxidizing agents , acids or water react violently. Magnesium phosphide releases toxic monophosphine even when it comes into contact with humidity :
Magnesium phosphide crystallizes in a cubic crystal structure with the space group Ia 3 (space group number 206) and the lattice parameter a = 12.01 Å . Similar to the Na 2 O structure, the structure consists of a distorted, cubic close-packed spherical packing of phosphide ions, in which three quarters of all tetrahedral gaps are occupied by magnesium ions.
use
The property that magnesium phosphide releases monophosphine even when it is damp is used to combat voles or protect plants. The gas penetrates the caves and passages of the animals and kills them there.
Magnesium phosphide can also be used for the laboratory preparation of monophosphine, but the gas thus obtained mostly ignites by itself due to traces of diphosphine in the air.
safety instructions
Magnesium phosphide is very toxic if swallowed, as gastric acid also creates toxic monophosphine. Furthermore, the resulting gases can ignite by themselves, which is why you should not add water to accelerate the gas development.
Individual evidence
- ↑ a b M. von Stackelberg, R. Paulus: Investigations on the crystal structure of the nitrides and phosphides of divalent metals. In: Journal for Physical Chemistry, Department B: Chemistry of Elementary Processes , Structure of Matter , 22, 1933, pp. 305–322, doi: 10.1515 / zpch-1933-2226 .
- ↑ a b c d e f Entry on magnesium phosphide in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Trimagnesium diphosphide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ agrosanita: EG-SDB - Degesch Plate ( Memento of the original from March 4, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 661 kB).
- ↑ Patent DE2945647A1 : Process for the production of aluminum phosphide and / or magnesium phosphide. Registered on November 12, 1979 , published on May 21, 1981 , Applicant: Degesch , Inventor: Franziskus Horn, Ekkehard Fluck.




