Hydrogen sulfide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Hydrogen sulfide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | H 2 S | |||||||||||||||
| Brief description |
colorless gas that smells of rotten eggs |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 34.08 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
−85.7 ° C |
|||||||||||||||
| boiling point |
−60.2 ° C |
|||||||||||||||
| Vapor pressure |
1.82 M Pa (20 ° C) |
|||||||||||||||
| pK s value |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Dipole moment | ||||||||||||||||
| Refractive index |
1.307 (16.85 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 5 ml m −3 or 7.1 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Hydrogen sulfide (also hydrogen sulfide , dihydrogen sulfide , not to be confused with the hydrogen sulfide anion HS - which is often also called "hydrogen sulfide") is a chemical compound of sulfur and hydrogen with the formula H 2 S. Hydrogen sulfide is a foul-smelling, colorless, highly toxic Gas . It is corrosive , flammable, highly flammable, and slightly heavier than air. It is not very soluble in water and somewhat more soluble in ethanol . H 2 S is a weak acid , the salts of which are sulfides and hydrogen sulfides.
Even in very low concentrations, hydrogen sulfide can be recognized by its typical rotten egg smell . He arises u. a. in the breakdown of sulfur-containing amino acids in the proteins of egg whites and yolks .
Occurrence
In nature, hydrogen sulfide occurs as a very variable component (from traces up to 80 vol%) in natural gas and in crude oil , as volcanic gas and dissolved in spring water . It also arises from the breakdown of biomass through putrefaction or putrefaction (e.g. animal carcasses, corpses, decomposition of leaf litter , sludge formation at the bottom of eutrophic lakes, etc.) or during digestive processes in the intestine , which it leaves with the flatus . The hydrogen sulphide that is produced during such biomass degradation in landfills , manure pits , high-pressure sewage pipes or other technical facilities causes billions in damage to concrete structures (biogenic sulfuric acid corrosion). One cause of the unpleasant halitosis in humans is - in addition to other volatile organic compounds containing sulfur ( methanethiol , dimethyl sulfide ) - hydrogen sulfide.
Extraction and presentation
Hydrogen sulphide can be produced on a laboratory scale by dripping hydrochloric acid onto iron (II) sulphide in the Kipp apparatus :
- Iron (II) sulfide and hydrochloric acid produce iron (II) chloride and hydrogen sulfide.
However, the resulting product is contaminated by the starting materials with gases such as hydrogen , carbon dioxide , nitrogen and oxygen . When using natural iron sulfide (e.g. pyrrhotite ), the product can also be contaminated with gases such as arsine , monophosphine , hydrogen selenide , hydrogen telluride and the like. Pure hydrogen sulfide can be obtained by heating a concentrated solution of magnesium hydrogen sulfide or from the elements, including sodium sulfide and phosphoric acid .
In the petrochemical industry ( refineries ), hydrogen sulfide is produced in large quantities during the hydrodesulfurization of petroleum .
properties
Physical Properties
- critical temperature : 100.15 ° C
- critical pressure : 89.7 bar
Thermodynamics:
S 0 g, 1 bar : 205.77 J / (mol K)
Up to 2.582 liters of hydrogen sulfide gas dissolve in 1 liter of water at room temperature .
Hydrogen sulfide is slightly heavier than air , under normal conditions the difference in density is about 19%.
Hydrogen sulfide liquefies under a pressure of 18.2 bar at room temperature. At 90 GPa, hydrogen sulfide is said to change into a metallic phase; H 3 S is assumed, which becomes type II superconducting at 203 K (−70 ° C) .
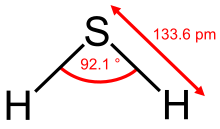
The H 2 S molecule is angled. The bond angle is 92.1 ° and the bond length is 133.6 pm. Because of the small electronegativity difference between the binding partners and thus the low binding polarity , hydrogen bonds do not play an essential role in hydrogen sulfide, which is expressed in the relatively low melting and boiling point .
Chemical properties
With a pK s value of 6.9 is hydrogen sulphide - as hydrosulfite - a rather weak acid . The aqueous solution reacts with many heavy metal salts to form insoluble sulfides , which is used in the cation separation process. Correspondingly, the gas is detected with lead acetate paper as it reacts with lead (II) ions to form black lead (II) sulfide . It also reacts with iron (II) ions to form black iron (II) sulfide .
The above recovery reaction is also reversible. Under natural conditions (pH 5–10), hydrogen sulfide can be bound in aqueous solution with iron (II) chloride to iron (II) sulfide .
This is common practice with biogas , digester gas and in sewers . The great affinity of iron to sulfur is used to purify biogas and digester gas. If it were to be used in gas engines, the sulfur dioxide produced after combustion would cause considerable corrosion problems.
When air is supplied, hydrogen sulfide burns with a blue flame to form sulfur dioxide and water , with the formation of sulfurous acid , among other things . When air is supplied, sulfur gradually separates out of the aqueous solution .
In the presence of water vapor, it proportions with sulfur dioxide to form sulfur and water ( flue gas desulfurization , redox reaction ); with chlorine , sulfur and hydrogen chloride gas are formed . Hydrogen sulfide is also a powerful reducing agent .
Hydrogen sulfide is moderately soluble in water . The aqueous solution is called hydrogen sulfide water. Hydrogen sulfide is partially subject to dissociation in the aqueous solution :
The possibility of influencing this dissociation process through the choice of the pH value of the solution , ie being able to set the sulfide ion concentration arbitrarily, is the basis for the analytical separation of the cations after the H 2 S separation process. While the extremely low concentration of sulfide ions in acidic solution is sufficient to precipitate the particularly poorly soluble sulfides of the elements of the H 2 S group, the solubility products of the slightly more soluble sulfides of the elements of the NH 4 HS group are only achieved in ammonia solution . In the air , hydrogen sulfide burns to form sulfur or sulfur dioxide ( Claus process ), depending on the prevailing conditions . With fluorine it reacts to hydrogen fluoride and sulfur hexafluoride :
With the other halogens, e.g. B. with chlorine, it reacts to form hydrogen halide and sulfur:
Safety-related parameters
Hydrogen sulphide forms highly flammable gas-air mixtures. The explosion range is between 4.3% by volume (60 g / m 3 ) as the lower explosion limit (LEL) and 45.5% by volume (650 g / m 3 ) as the upper explosion limit (UEL). The maximum explosion pressure is 5.9 bar. The limit gap width was determined to be 0.83 mm. This results in an assignment to explosion group IIB. The ignition temperature is 270 ° C. The substance therefore falls into temperature class T3.
use
Large chemistry
Hydrogen sulfide is the main source of elemental sulfur , which in turn is converted to more than 95 percent into sulfuric acid. Part of the hydrogen sulfide is burned to sulfur dioxide :
Part of the remaining hydrogen sulphide reacts with the sulfur dioxide formed by comproportioning to form elemental sulfur:
Chemical analysis
In the classic cation separation process , it is used to precipitate an entire group ( hydrogen sulfide group ). By introducing H 2 S gas into weakly acidic solutions, the following precipitates: As 2 S 3 , SnS 2 , Sb 2 S 3 , HgS , SnS , PbS , Bi 2 S 3 , CuS and, when diluted with water, also CdS . These cations then have to be separated further and identified with the help of detection reactions.
Because of its toxicity, hydrogen sulfide is increasingly being dispensed with in the cation separation process. Instead, the required sulfide anions are generated in situ , for example with the help of thioacetamide , in smaller quantities also by heating sulfur with candle wax.
H 2 S-Gang : This procedure builds on the classic separation walk . Chemically similar cations are precipitated in groups using specific reagents. The precipitate is then separated and analyzed, the supernatant (the solution) is used and the next group is precipitated.
toxicology
toxicity
Hydrogen sulfide is an extremely poisonous gas that can cause hydrogen sulfide poisoning. Due to its higher density than air, the gas collects on the ground.
Hydrogen sulphide has the property of numbing the odor receptors , so that an increase in concentration is no longer perceived through the odor. The threshold for anesthetizing the odor receptors is at a concentration of 200 ppm H 2 S. The intense, easily perceptible odor of rotten eggs is therefore an invaluable advantage over other deadly gases, but in case of doubt it is deceptive.
Short-term toxic effect
When it comes into contact with mucous membranes and tissue fluid in the eyes, nose, throat and lungs, the hydrogen sulphide forms alkali sulphides, which cause a very strong irritation. One consequence of this is water retention in the lungs. Symptoms usually go away within a few weeks.
The actual toxic effect is based on the destruction of the red blood pigment hemoglobin and thus a paralysis of the intracellular respiration. The mechanism is still unclear, it is assumed that oxygen- transmitting enzymes containing heavy metals are inactivated. The smaller, non-oxidized part of the hydrogen sulfide can cause damage to the central and possibly also the peripheral nervous system .
The following effects occur on humans :
- from 20 ppm: corneal damage with prolonged exposure
- ≈ 100 ppm: irritation of the mucous membranes of the eyes and airways , salivation , irritation of the throat
- > 200 ppm: headache , difficulty breathing
- > 250 ppm: stunning of the olfactory receptors
- > 300 ppm: nausea
- ≈ 500 ppm: weakness, drowsiness, dizziness
- > 500 ppm: convulsions , loss of consciousness
Long-term exposure to low doses can lead to tiredness , loss of appetite , headache , irritability , poor memory and poor concentration .
Concentration- dependent symptoms of poisoning arise in humans :
- <100 ppm: after several hours
- > 100 ppm: <1 hour
- ≈ 500 ppm: life threatening in 30 minutes
- ≈ 1000 ppm: life threatening in a few minutes
- ≈ 5000 ppm (corresponds to a volume fraction of 0.5%): fatal in a few seconds
This means that H 2 S concentrations as low as 0.1% are fatal after a few minutes. At such concentrations, unconsciousness occurs within one or more breaths.
The concentration of H 2 S, which in human cells by inhibiting the cellular respiration is harmful, was in vitro with 0.32 μ mol / l determined.
Long-term effect
Animal studies show that pigs fed foods containing hydrogen sulfide suffer from diarrhea after a few days and show weight loss after about 105 days.
physiology
metabolism

Hydrogen sulphide is formed in the body for a short time when an excess of cysteine is broken down by means of cystathionine γ-lyase ( EC 4.4.1.1 ), which normally breaks down cystathionine to cysteine, but can also break down cysteine further:
Another reaction of the same enzyme was found in rats, which originates from cystine , but plays no role in humans:
The gas quickly combines with thiol residues from surrounding proteins (-Cys becomes -CySSH) and thereby changes their biological activity. In particular, the enzyme cytochrome c oxidase is deactivated. The majority, however, is oxidized to sulfate in the mitochondria via thiosulfate and sulfite, or processed to sulfite / sulfate or taurine via cysteine sulfinate.
Oxidation to sulphate
Mitochondria protect themselves from H 2 S or HS - through its oxidation to sulfate, which takes place in three steps:
First, H 2 S is oxidized to thiosulfate by an enzyme complex. In detail, three individual reactions take place, which are catalyzed by the enzymes sulfide: quinone oxidoreductase ( EC 1.8.5.- ), sulfur dioxidease ( EC 1.13.11.18 ) and rhodanase .
Part of the oxidation of thiosulphate to sulphite takes place with the help of glutathione and the enzyme thiosulphate reductase ( EC 2.8.1.3 ), another part uses thiosulphate sulfur transferase .
Finally, the sulfite oxidase oxidizes sulfite to sulfate. A confirmation of the degradation pathway resulted from the identification of the mitochondrial Schwefeldioxygenase with the ETHE1 - gene , which in a rare mutation in a genetic disease with increased damage caused by H 2 S concentrations leads.
function
In the human body, hydrogen sulfide acts like nitric oxide as a messenger substance (see also gasotransmitter ) and has a vasodilator effect . It is produced from the amino acid L - cysteine in endothelial cells of the blood vessels as well as in smooth muscle cells . If the vascular endothelium is stimulated via muscarinic acetylcholine receptors, H 2 S is released. This leads to the activation of voltage-activated and calcium-activated potassium channels in the smooth muscle cells of the vascular muscles . This leads to a hyperpolarization of the smooth muscle cells and ultimately to an expansion of the blood vessels (vasodilation) .
application
Hydrogen sulfide could potentially be used as a remedy for erectile dysfunction. It is naturally formed in the cavernous bodies of the penis and the smooth muscles of the penile artery . Tests have shown that both L- cysteine and hydrogen sulfide (salt) supplied externally cause a concentration-dependent erection in the erectile tissue of the penis ( Corpora cavernosa penis ).
In low concentrations, hydrogen sulfide slows down metabolic processes and lowers body temperature in mice. This hibernation-like state is fully reversible and harmless to the animals. Investigations are ongoing as to whether this effect can be used in transplant medicine to improve the quality and survival time of organs intended for transplantation. In addition, human studies are being carried out to determine whether hydrogen sulfide can improve the likelihood of survival in emergency patients. The aim is to slow down the metabolism by inhaling or injecting H 2 S and thus to reduce the need for oxygen. Ideally, this measure would already be preclinical, e.g. B. by the emergency services .
In a 2007 study at the University of Alabama at Birmingham , which was published in the journal Proceedings of the National Academy of Sciences , hydrogen sulfide in very low doses is also believed to be a major factor in garlic's health effects . The authors report that garlic lowers the risk of heart disease from high blood pressure, increased blood fat ( cholesterol ), and other factors. In population groups that consume a lot of garlic, there are therefore fewer problems with high blood pressure.
Analytics
Both the toxicity and its biological relevance place high demands on the analysis of hydrogen sulfide. In contrast to the above-mentioned use of H 2 S in the inorganic separation process, instrumental, quantitative detection methods for H 2 S are presented here.
Instrument-based analytics
Optical determination
The most frequently used chromogenic reaction for the photometric detection of H 2 S and sulfides is the reaction with N ', N-dimethyl-p-phenylenediamine to form methylene blue . Iron (III) salts are used as a catalyst. The reaction product has an absorption maximum at 670 nanometers and can be determined photometrically.
Electroanalysis
Amperometry
Amperometric H 2 S sensors are widely used . In amperometry, a potential is applied to a working electrode and the resulting current is measured; this is proportional to the concentration of H 2 S. Hydrogen sulfide is oxidized to sulfate. Electrodes modified with carbon nanotubes achieved a detection limit of 0.3 µmol / l at an oxidation potential of 100 mV. The design of the electrodes used is closely related to that of the Clark electrode for oxygen determination.
Potentiometry
Potentiometric probes have also been developed for the sensing of gaseous H 2 S. As an example, solid electrolyte-based, galvanic half-cells can be mentioned, which together with H 2 S deliver an electromotive force that is measured. With yttrium oxide-stabilized zirconium tubes as sensors, H 2 S concentrations in air of up to 0.2 ppm could be measured with reliable reproducibility. Using hexacyanoferrate as a redox partner, even 30 ppb H 2 S could be detected.
Gas chromatography
Gas chromatography is often the first choice for the analysis of gaseous substances . After separation, sulfur compounds such as H 2 S can be detected by flame photometry at an emission wavelength of 397 nanometers. One method for the rapid detection of traces of hydrogen sulfide in coal gas achieved a detection limit of 10 ppb.
See also
literature
- Dieter Weismann, Manfred Lohse (Hrsg.): Sulfide practical handbook of waste water technology. Vulkan, Essen 2007, ISBN 978-3-8027-2845-7 .
- Tatjana Hildebrandt, Manfred K. Grieshaber: The many sides of the sulfide. Deadly and yet vital. In: Biology in our time 39, No. 5, 2009, pp. 328–334, doi: 10.1002 / biuz.200910403 .
- FP Springer: About sulfur and hydrogen sulfide - the history of these components of natural gas. Erdöl-Erdgas-Kohl, Issue 10, 2011, pp. 382–388.
Web links
- baua.de: Leaflet for BK No. 1202: Diseases caused by hydrogen sulfide
- Underwater River in Mexico. In: Visboo. November 17, 2009 (photos of a visible accumulation of hydrogen sulfide at the bottom of a cave)
- Current page only about hydrogen sulfide
Individual evidence
- ↑ a b c d e f g h i entry to hydrogen sulfide in the GESTIS database of IFA , retrieved on February 1, 2016 (JavaScript required)
- ↑ a b Entry on hydrogen sulfide. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b chem.wisc.edu: pKa Data , Compiled by R. Williams (PDF, 78 kB).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Dipole Moments, pp. 9-51.
- ↑ PG Sennikov, VE Shkrunin, DA Raldugin, KG Tokhadze: Weak Hydrogen Bonding in Ethanol and Water Solutions of Liquid Volatile Inorganic Hydrides of Group IV – VI Elements (SiH 4 , GeH 4 , PH 3 , AsH 3 , H 2 S, and H 2 Se). 1. IR Spectroscopy of H Bonding in Ethanol Solutions in Hydrides . In: The Journal of Physical Chemistry . tape 100 , no. 16 , 1996, pp. 6415-6420 , doi : 10.1021 / jp953245k .
- ↑ Entry on Hydrogen sulphide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on November 2, 2015.
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) pp. 61–62, ISBN 978-3-8348-1245-2 .
- ↑ Georg Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. Volume 1. 2nd edition. Academic Press, New York 1963, pp. 344-346.
- ↑ a b A.G. Cubitt et al .: Some thermodynamic properties of liquid hydrogen sulphide and deuterium sulphide in J. Chem. Thermodyn. 19 (1987) 703.
- ^ AP Drozdov et al .: Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system . Nature Letter 525, 2015, pp. 73–76, doi: 10.1038 / nature14964 .
- ↑ Manfred Lindinger: The perfect conductor for arctic cold . FAZ August 21, 2015.
- ↑ a b Spectrum of Science Verlagsgesellschaft mbH: Hydrogen sulfide
- ↑ Elisabeth Brandes, Wolfgang Möller: Flammable liquids and gases. Wirtschaftsverlag NW, Bremerhaven 2003, ISBN 3-89701-745-8 ( Safety-related parameters. Volume 1).
- ↑ Jürgen Falbe, Manfred Regitz (Ed.): Römpp Chemie-Lexikon. Thieme, Stuttgart 2008.
- ^ RJ Reiffenstein, WC Hulbert, SH Roth: Toxicology of hydrogen sulfide. In: Annual Review of Pharmacology and Toxicology Volume 32, 1992, pp 109-134. doi: 10.1146 / annurev.pa.32.040192.000545 , PMID 1605565 (review).
- ↑ M. Goubern, M. Andriamihaja u. a .: Sulfide, the first inorganic substrate for human cells. In: The FASEB journal : official publication of the Federation of American Societies for Experimental Biology Volume 21, Number 8, June 2007, pp. 1699–1706. doi: 10.1096 / fj.06-7407com . PMID 17314140 .
- ↑ a b c M. H. Stipanuk, I. Ueki: Dealing with methionine / homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. In: Journal of inherited metabolic disease Volume 34, Number 1, February 2011, pp. 17-32. doi: 10.1007 / s10545-009-9006-9 . PMID 20162368 . PMC 290177 (free full text). (Review).
- ↑ TM Hildebrandt, MK Grieshaber: Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. In: The FEBS Journal Volume 275, Number 13, July 2008, pp. 3352-3361. doi: 10.1111 / j.1742-4658.2008.06482.x . PMID 18494801 .
- ↑ Rui Wang: Hydrogen sulfide: a new EDRF . In: Kidney International . tape 76 , no. 7 , 2009, p. 700-704 , doi : 10.1038 / ki.2009.221 .
- ^ Roberta d'Emmanuele di Villa Biancaa u. a .: Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. In: Proceedings of the National Academy of Sciences . 106, No. 11, 2009, doi: 10.1073 / pnas.0807974105 .
- ↑ Eric Blackstone, Mike Morrison, Mark B. Roth: H2S Induces a Suspended Animation-Like State in Mice . In: Science . tape 308 , no. 5721 , 2005, p. 518 , doi : 10.1126 / science.1108581 .
- ↑ Eelke M. Bos u. a .: Hydrogen Sulfide-Induced Hypometabolism Prevents Renal Ischemia / Reperfusion Injury . In: Journal of the American Society of Nephrology . tape 20 , no. 9 , 2009, p. 1901–1905 , doi : 10.1681 / ASN.2008121269 .
- ↑ Ikaria: Pipeline ( Memento from June 21, 2009 in the Internet Archive )
- ↑ Mark Roth: Suspended animation is within our grasp (English)
- ↑ Gloria A. Benavides et al. a .: Hydrogen sulfide mediates the vasoactivity of garlic. In: Proceedings of the National Academy of Sciences. 104, No. 46, 2007, pp. 17977-17982, doi: 10.1073 / pnas.0705710104 .
- ↑ B. Lange, ZJ Vejdelek: Photometric analysis. Verlag Chemie, Weinheim 1980, ISBN 3-527-25853-1 .
- ↑ NS Lawrence, R. P. Deo, J. Wang; Electrochemical determination of hydrogen sulfide at carbon nanotube modified electrodes In: Analytica Chimica Acta . 517, No. 1-2, 2004, pp. 131-137, doi: 10.1016 / j.aca.2004.03.101 .
- ↑ P. Jeroschewski, C. Steuckart, M. Kühl: An Amperometric Microsensor for the Determination of H 2 S in Aquatic Environments. In: Analytical Chemistry . 68, No. 24, 1996, pp. 4351-4357, doi: 10.1021 / ac960091b .
- ↑ N. Miura, Y. Yan, G. Lu, N. Yamazoe; Sensing characteristics and mechanism of hydrogen sulfide sensor using stabilized zirconia and oxide sensing electrode. In: Sensors and Actuators B: Chemical . 34, No. 1-3, 1996, pp. 367-372, doi: 10.1016 / S0925-4005 (96) 01828-X .
- ↑ P. Jeroschewski, K. Haase, A. Trommer, P. Gruendler: Galvanic sensor for determination of hydrogen sulfide. In: Electroanalysis . 6, No. 9, 2005, pp. 769-772, doi: 10.1002 / elan.1140060910 .
- ↑ T. Ubuka, T. Abe, R. Kajikawa, K. Morino: Determination of hydrogen sulfide and acid-labile sulfur in animal tissues by gas chromatography and ion chromatography. In: Journal of Chromatography B: Biomedical Sciences and Applications . 757, No. 1, 2001, pp. 31-37, doi: 10.1016 / S0378-4347 (01) 00046-9 .
- ↑ JB Laurens, ER Rowher; High Speed Analysis of H 2 S and COS Impurities in Coal Gas by Capillary Gas Chromatography. In: Journal of High Resolution Chromatography . 19, No. 4, 1996, pp. 217-223, doi: 10.1002 / jhrc.1240190408 .
- ↑ M. Jiang, P. Ning, Y. Shi: Determination of Hydrogen Sulfide in Waste Gas by Gas Chromatography. In: Journal of Environment and Health. 25, 2008 pp. 1088-1089 ( abstract ).