Gas transmitter
Gasotransmitters are gaseous substances under standard conditions that are synthesized in cells and play an important role in intercellular communication. These include nitrogen monoxide (NO), hydrogen sulfide (H 2 S), carbon monoxide (CO) and, if necessary, nitrous oxide (laughing gas N 2 O).
General
Gasotransmitters belong to a family of endogenous gas molecules or gaseous signal molecules , such as NO, CO, H 2 S, and others. These gases have many features in common, for example in their cellular production and function, and their biological tasks are also characterized in a unique way, which distinguishes them from classical signaling molecules. Their distribution is ubiquitous from unicellular to multicellular organisms, they occur in all domains . From a phylogenetic point of view, they are very old cellular communication principles.
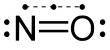


For the first time in 1981 a clinical work pointed to the pharmacological effects of gasotransmitters on corresponding receptors and as neurotransmitters .
An in vitro - experiment confirmed these observations. A binding terminology and characterization for the criterion "gasotransmitter" was introduced in 2002.
A gas can be categorized as a gas transmitter if its molecules meet the following criteria:
- You are small.
- They are freely permeable , permeable to biological membranes . They have endocrine , paracrine and autocrine effects. In their endocrine mode of action, for example, gasotransmitters can be released from tissues, spread in the bloodstream and then develop modulating functions on distant target cells.
- They are produced endogenously and enzymatically and their production is regulated .
- They are chemically defined and have specific functions in physiologically relevant concentrations . Thus, in endogenous concentrations, they cause specific physiological changes.
- Their physiological and cellular functions can be mimicked by exogenously applied molecules.
Biological synthesis and effects of nitric oxide (NO)
NO is produced enzymatically through the action of the various NO synthases (NOS so NOS-I, NOS-II and NOS-III) from the amino acid L-arginine .
The small molecule NO is chemically highly reactive and thus destabilizing for the biochemical structures of cells. From an evolutionary point of view, this makes precise regulation of NO production useful. Two main principles of the mechanism of action of NO are described:
- NO acts on metalloproteins , more precisely on metal ions in the center of these proteins , e.g. B. of enzymes, and increases or decreases their activity ,
- NO causes protein nitrosylation , whereby the NO molecule is bound to a - SH - or - S – CH 3 group on its sulfur atom. This triggers a change in conformation and, as a result , a change in function in the protein.
Released NO acts intracellularly via the activation of the soluble guanylyl cyclases and, as a result, with an increase in intracellular production of the second messenger cyclic guanosine monophosphate (cGMP).
Biological synthesis and effects of carbon monoxide (CO)
About 86% of the CO molecule is formed in the organism by the oxidative breakdown from the heme molecule with the simultaneous release of iron (Fe 2+ or then to ferritin ) and biliverdin .
Heme b + 3O 2 + 3½NADPH + 3½H + + 7e- → biliverdin + Fe 2+ + CO + 3½NADP + + 3H 2 O

Only 14% of the endogenous CO comes from photooxidation, lipid peroxidation and bacteria.
The heme-degrading enzyme heme oxygenase is found in almost all animal tissues, in vertebrates mainly in the spleen . According to the current state of research, three isoforms are known, two of them in humans.
- The inducible HO-1 (also referred to as heat shock protein 32 ) can be upregulated using various substances; these are for example the heme molecule ( positive feedback ), various heavy metals, growth factors , the NO molecule, various lipids, in the context of hypoxia .
- The constitutive HO-2 in various organs or tissues of these organs, such as the central nervous system, endothelium and the testes .
The CO molecule can also activate soluble guanylate cyclase and trigger its intracellular effects through increased production of intracellular cGMP. The CO molecule influences the cGMP to a lesser extent than the NO molecule, so activation by CO is only a factor of 4–5; in comparison, NO causes a 200-fold activation.
Biological synthesis of hydrogen sulfide (H 2 S)
Hydrogen sulfide is synthesized in the cells from the amino acid L- cysteine by the action of the enzyme cystathionine-γ-lyase (CSE) ( EC 4.4.1.1 ) or the cystathionine-β synthase (CBS).
Cysteine + H 2 O → serine + H 2 S
Once released, the gas reacts quickly with thiol residues from the surrounding proteins (-Cys becomes -CySSH) and thereby changes their biological activity ( conformational change ). In particular, the enzyme cytochrome c oxidase is deactivated. The majority, however, is oxidized to sulfate in the mitochondria via thiosulfate and sulfite , or processed to sulfite / sulfate or taurine via cysteine sulfinate. Enzymes that are able to produce H 2 S are found in various types of tissue or organs , for example in the blood vessel system, in the liver, in the kidneys and in the brain. The highest H 2 S concentrations are also found in the latter organ .
literature
- Ashley A. Untereiner, Lingyun Wu, Rui Wang: The Role of Carbon Monoxide as a Gasotransmitter in Cardiovascular and Metabolic Regulation. In: A. Hermann et al. (Ed.): Gasotransmitters: Physiology and Pathophysiology. Springer-Verlag, Berlin / Heidelberg 2012, ISBN 978-3-642-30338-8 , pp. 37-70.
- C. Ori, F. Ford-Rice, ED London: Effects of nitrous oxide and halothane on μ- and κ-opioid receptors in guinea-pig brain. In: Anesthesiology. 70, 1989, pp. 541-544.
- MA Gillman: Nitrous oxide as neurotransmitter. In: Lancet. 339, 1992, p. 307.
- MA Gillman: Nitrous oxide, Nitric oxide and neurotransmission. In: Brit Med J. 305, 1992, p. 1368.
- MA Gillman, FJ Lichtigfeld: NO comments. In: Nature. 367, 1994, p. 28.
- MA Gillman: Discovery of gasotransmission. In: The Scientist. 18 (2004)
- J. Hyun, G. Chaudhuri, JM Fakuto: The reductive metabolism of nitric oxide in hepatocytes: possible interaction with thiols. In: Dru. Metab Dispos. 27, 1999, pp. 1005-1009.
- O. Einarsdottir, WS Caughey: Interactions of the anesthetic N 2 O with bovine heart cytochrome c oxidase. In: J Biol Chem. 263, 1988, pp. 9199-9205.
- R. Wang (Ed.): Signal Transduction and the Gasotransmitters: NO, CO and H 2 S in Biology and Medicine. Humana Press, New Jersey, USA (2004)
- R. Wang: Two's company, three's a crowd - Can H 2 S be the third endogenous gaseous transmitter? In: FASEB Journal. 16, 2002, pp. 1792-1798.
- JP Cooke: The 1998 Nobel prize in Medicine: clinical implications for 1999 and beyond. In: Vascular Medicine. 4, 1999, pp. 57-60.
- J. Garthwaite: Concepts of neural nitric oxide-mediated transmission. In: European Journal of Neuroscience. 27, 2008, pp. 2783-2802.
- A. Papapetropoulos, A. Pyriochou, Z. Altaany, G. Yang, A. Marazioti, Z. Zhou, MG Jeschke, LK Branski, DN Herndon, R. Wang, C. Szabó: Hydrogen sulfide is an endogenous stimulator of angiogenesis. In: PNAS. 2009.
- Friedrich Marks, Ursula Klingmüller, Karin Müller-Decker: Cellular Signal Processing. An Introduction to the Molecular Mechanisms of Signal Transduction. Taylor & Francis, Garland Science 2009, ISBN 978-0-8153-4215-1 , pp. 580-585.
- Julia Steidle: Effects of the gasotransmitter carbon monoxide on ion transport in the rat's colon. Inaugural dissertation . Department of Veterinary Medicine at the Justus Liebig University in Giessen. VVB Laufersweiler Verlag, Giessen 2011. (PDF file; 5.74 MB)
Web links
- gasotransmitters.eu: European Network on Gasotransmitters (ENOG) , official website
- Verena Engelke; Adina Rocher; Peter Imming: Gasotransmitter. Paradox carbon monoxide. Pharmaceutical newspaper online, issue 33/2010, online 2012
- A. Hermann et al.: Gases as cellular signal substances. Gas transmitter. (PDF file; 1.48 MB) Biology in Our Time 2010; 40, pp. 185-193. doi: 10.1002 / biuz.201010422
- SpringerMedizin.at: Gasotransmitter: volatile transmitter substances , October 19, 2010
Individual evidence
- ^ A b Anton Hermann, Guzel F. Sitdikova, Thomas M. Weiger: Gases as cellular signal substances. Gas transmitter. In: Biology in Our Time. 40, 2010, pp. 185–193, doi: 10.1002 / biuz.201010422 .
- ^ MA, Gillman, FJ Lichtigfeld: A comparison of the effects of morphine sulphate and nitrous oxide analgesia on chronic pain states in man. In: J. Neurol. Sci. 49 (1), 1981, pp. 41-45. doi: 10.1016 / 0022-510X (81) 90186-6 . PMID 7205318 .
- ^ MA Gillman, FJ Lichtigfeld: The similarity of the action of nitrous oxide and morphine. In: Pain. 10 (1), 1981, p. 110. PMID 7232008 .
- ^ MA Gillman, FJ Lichtigfeld: Nitrous oxide interacts with opioid receptors: more evidence. In: Anesthesiology. 58 (5), 1983, pp. 483-484. PMID 6301312 .
- ↑ C. Daras, R. Cantrill, MA Gillman: (3H) Naloxone displacement: evidence for nitrous oxide as opioid receptor agonist. In: Eur J Pharmacol. 89, pp. 177-178.
- ↑ a b R. Wang: Two's company, three's a crowd - Can H 2 S be the third endogenous gaseous transmitter? In: FASEB Journal. 16, 2002, pp. 1792-1798.
- ↑ R. Wang (Ed.): Signal Transduction and the Gasotransmitters: NO, CO and H 2 S in Biology and Medicine. Humana Press, New Jersey, USA 2004.
- ^ RG Knowles, S. Moncada: Nitric oxide synthases in mammals. In: Biochem J. 298, 1994, pp. 249-258.
- ↑ Verena Engelke, Adina Rocher, Peter Imming: Gasotransmitter. Paradox carbon monoxide. Pharmaceutical newspaper, 33/2010 [1]
- ↑ SJ Gibbons; G. Farrugia: The role of carbon monoxide in the gastrointestinal tract. In: J Physiol. 556, 2004, pp. 325-336.
- ↑ MH Stipanuk, I. Ueki: Dealing with methionine / homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. In: Journal of inherited metabolic disease. Volume 34, Number 1, February 2011, pp. 17-32. doi: 10.1007 / s10545-009-9006-9 . PMID 20162368 . PMC 290177 (free full text).


