Heme oxygenase
| Heme oxygenase | ||
|---|---|---|
|
||
|
Existing structural data: s. UniProt |
||
| Properties of human protein | ||
| Mass / length primary structure | 288 amino acids | |
| Identifier | ||
| Gene names | HMOX1 ; HMOX2 | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.14.99.3 , dioxygenase | |
| Response type | oxidation | |
| Substrate | Heme + 4 NADPH / H + + 3 O 2 | |
| Products | Biliverdin + Fe 2+ + 4 NADP + + CO + 3 H 2 O | |
| Occurrence | ||
| Homology family | HOX2 | |
| Parent taxon | Creature | |
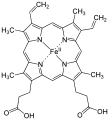
Hemoxygenase (HMOX) is the name for the enzyme that oxidizes and breaks down heme into iron (in the form of Fe 3+ ions), biliverdin and carbon monoxide . It occurs in mammals in the smooth endoplasmic reticulum of the cells, but also in cyanobacteria and red algae . Mutations in the HMOX gene in humans can very rarely cause haemoxygenase deficiency. The enzyme is not only indispensable for porphyrin degradation, but is also involved in signal transduction .
Heme oxygenase exists in mammals in up to three isoforms , in humans in two, the inducible isoform heme oxygenase-1 (HO-1) and the constitutive isoform heme oxygenase-2 (HO-2). Hemoxygenase-1 is a protein with a molar mass of 32 kDa . Hemoxygenase-1 is upregulated in mammalian tissues by a large number of stimuli, such as TGF-β , platelet-derived growth factor (PDGF) , vascular endothelial growth factor (VEGF) , stromal cell-derived factor 1 (SDF-1) , NO , peroxynitrite , lipid peroxides , oxygen deficiency (hypoxia) , oxidative stress , cytokines and others. Hemoxygenase-2 becomes constitutive, i.e. H. formed (expressed) in the brain , endothelium and testes independently of internal and external factors . The breakdown of heme by the heme oxygenase system is the main source of the formation of carbon monoxide in the body, which acts as a gas transmitter .
|
||
The heme oxygenase catalyzes the oxidation of heme to biliverdin with the release of iron ions and carbon monoxide. The enzyme works “region-specifically” on the heme molecule. Only the α-isomer of biliverdin is released, which is then broken down by the NADPH cytochrome P450 oxidoreductase to the α-isomer of bilirubin. The reaction is completed by the NADPH-dependent reduction of iron in the heme complex.
Biological importance
Hemoxygenase-1 and the metabolite carbon monoxide perform important functions in the body: They promote the formation of new blood vessels (proangiogenic effect) and inhibit inflammatory processes (anti-inflammatory effect) , oxidative stress (antioxidant effect) , increased connective tissue formation ( antifibrotic effect ) and programmed cell death (anti-apoptotic effect) . VEGF and SDF-1 exert their effect proangiogenetische is characterized in that it heme oxygenase induce . A massive number of preclinical and clinical studies describe the anti-inflammatory effects of the enzyme. According to individual investigations, however, HO-1 could also promote certain chronic inflammations and thus act as an interface between obesity and secondary diseases.
HO-1 in the intestinal mucosa breaks down the heme ingested with food.
Medical importance
Embryonic development
In embryonic development , the formation of new vessels and thus the heme oxygenase / carbon monoxide system is of crucial importance. In preeclampsia , the concentration of haem oxygenase-1 in the placenta is reduced, in the affected pregnant women the concentration of carbon monoxide in the exhaled air is reduced. Women who smoke who have high levels of carbon monoxide in their blood are less likely to develop preeclampsia. In preeclampsia, hemoxygenase-1 and carbon monoxide inhibit the release of antiangiogenic mediators such as Soluble fms-like tyrosine kinase-1 (sFlt1) and Soluble endoglin (sEng) .
Tumor angiogenesis
Various tumors , including renal cell carcinoma and prostate carcinoma, express high levels of heme oxygenase-1. Hemoxygenase promotes the formation of new blood vessels in tumors and inhibits the programmed cell death of tumor cells. In animal models, inhibition of heme oxygenase leads to a reduction in tumor growth.
Wound healing
The formation of new vessels ( neovascularization ) is a prerequisite for wound healing . Mice with reduced formation of heme oxygenase-1 show impaired wound healing .
diabetes
The enzyme heme oxygenase-1 (HO-1) has attracted attention in research on type 2 diabetes . It is present in significant amounts when the body is stressed or sick. Regular exercise and proper nutrition would not cause HO-1 to rise in disease-causing amounts. According to this, lack of exercise increases the level of HO-1, which worsens the state of health and in turn increases HO-1 production.
One study suggested high HO-1 levels as a predictor of metabolic syndrome .
Connective tissue formation
Hemoxygenase-1 inhibits a pathological increase in connective tissue ( fibrosis ). Mice with reduced formation of hemoxygenase-1 show increased fibrosis, increased expression of TGF-β1 , an increased inflammatory reaction and an increased transition from epithelial cells to connective tissue cells ( epithelial-mesenchymal transition (EMT ) when urinary outflow is impeded ( urinary tract obstruction ) in the affected kidneys ) ) on. Induction of heme oxygenase-1 by hemin inhibits renal fibrosis via an anti-apoptotic pathway; Zinc protoporphyrin , an inhibitor of hemoxygenase-1, partially eliminates this antifibrotic effect.
literature
- J Dulak et al .: Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis . In: Circulation . Jan 15, 117 (2), 2008, pp. 231-241 , PMID 18195184 ( ahajournals.org ).
Web links
Individual evidence
- ↑ Verena Engelke, Adina Rocher, Peter Imming: Gasotransmitter. Paradox carbon monoxide. Pharmaceutical newspaper, 33/2010 [1]
- ↑ Motterlini, R. and LE Otterbein (2010). "The therapeutic potential of carbon monoxide." Nature Reviews Drug Discovery 9 (9): 728-U724
- ↑ Pospisilik J. Andrew et al. HO-1 makes fat people sick , Max Planck Society, July 3, 2014
- ↑ Ursula Biermann: MEDICINE - An enzyme as a possible trigger of diabetes , in Deutschlandfunk " Forschung aktuell " from July 9, 2014
- ↑ Jais A u. a .: Heme Oxygenase-1 Drives Metaflammation and Insulin Resistance in Mouse and Man , Cell , Volume 158, No. 1, pp. 25-40, July 2014
- ↑ JH Kie et al .: Heme Oxygenase-1 Deficiency Promotes Epithelial-Mesenchymal Transition and Renal Fibrosis . In: J Am Soc Nephrol . No. 19 , p. 1681-1691 , PMID 18495963 .
- ↑ JH Kim et al .: Heme oxygenase-1 protects rat kidney from ureteral obstruction via an antiapoptotic pathway . In: J Am Soc Nephrol . 17 (5), May, 2006, pp. 1373-1381 , PMID 16597687 ( article ).