NADPH cytochrome P450 oxidoreductase
| NAPDH cytochrome P450 oxidoreductase | ||
|---|---|---|
|
||
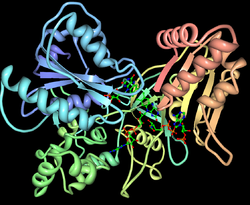
| Ribbon model of NAPDH cytochrome P450 reductase (from yeast). The structure is the result of the fusion of two proteins: flavodoxin (blue) and ferredoxin: NADP + reductase (red / yellow). | ||
| Properties of human protein | ||
| Mass / length primary structure | 680 amino acids | |
| Cofactor | FMN , FAD | |
| Isoforms | NP_000932.3 | |
| Identifier | ||
| Gene name | POR | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.6.2.4 , oxidoreductase | |
| Response type | Electron transfer | |
| Substrate | microsomal cytochrome P450 monooxygenases: CYP17, CYP19 | |
| Occurrence | ||
| Parent taxon | Bacteria , eukaryotes | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 5447 | 18984 |
| Ensemble | ENSG00000127948 | ENSMUSG00000005514 |
| UniProt | P16435 | P37040 |
| Refseq (mRNA) | NM_000941 | NM_008898 |
| Refseq (protein) | NP_000932 | NP_032924 |
| Gene locus | Chr 7: 75.92 - 75.99 Mb | Chr 5: 135.67 - 135.74 Mb |
| PubMed search | 5447 |
18984
|
NADPH-cytochrome P450 oxidoreductase (CPR) , (also NADPH hemoprotein reductase , gene name POR ) is the enzyme that the electron transfer from NADPH / H + to microsomal cytochrome P450 - monooxygenases (CYP17A1, CYP19A1) effected. CPR occurs in all living things (except in archaea ). In the membrane of the microsomes, CPR and CYP form a complex that absorbs the electrons from NADPH / H + and thereby enables the oxidation of pregnenolone, for example, to 17-hydroxy-pregnenolone with CYP17A1 , or the aromatization of testosterone to estradiol by the CYP19A1 .
An enzyme as a fusion protein
There are already three functions of CPR in bacteria: 1. Transfer of electrons from NADPH / H + by ferredoxin reductase ( FAD ), 2. transfer of electrons to ferredoxin / flavodoxin ( FMN ), 3. transfer of an electron to the Cytochrome P450 heme group . First of all, it is separate proteins, ferredoxin reductase and flavodoxin, that share the tasks. For POR , the genes are then fused together at some point.
However, fusion proteins of ferredoxin reductase and flavodoxin are also found in bacteria, which then also contain a cytochrome P450 enzyme, e.g. B. the P450BM3 from Bacillus megaterium or the fatty acid hydroxylases from Fusarium oxysporum or Bacillus subtilis . Bacterial CPR also contains the cytochrome P450 protein. Another similar fusion protein is also the human neuronal nitric oxide synthase nNOS, while three proteins are found in the cytochrome B5 system: NADH-cytochrome B5 reductase, cytochrome B5 a desaturase.
There are homologies to another flavoprotein, sulfite reductase.
Structure and function
Unlike nNOS, which is found in the cytosol, CPR is an endoplasmic reticulum protein . There it forms complexes with various CYP monooxygenase.
The only POR gene in animals is located on chromosome 7 gene locus q11.2 in humans . Plants have several gene copies.
The reactions of different diflavin reductases, the individual transfer reactions and their stereochemistry, and the interaction with NADPH have recently been elucidated.
POR genetic defects
In patients with adrenal disorders, malformations of the genital organs and bone malformations that correspond to Antley-Bixler syndrome , a genetic change in the POR gene is often observed. Since the gene product of POR is necessary for the activation of all microsomal P450 enzymes, mutations affecting these functions impair the activity of several CYP enzymes. In terms of steroid hormone production, CYP17, CYP21A2 and CYP19 are affected. Failure of CYP19 in particular leads to reduced virilization in male and increased virilization in female newborns. Androgens and estrogens are also important regulators for bone development and their failure impairs the development of a normal skeleton. Recessive POR mutations were observed in 50 different patients. also cytochrome POR deficiency . The extent to which POR defects also influence the metabolism of microsomal liver CYP monooxygenases has not yet been investigated.
Individual evidence
- ↑ a b c Murataliev et al. : Electron transfer by diflavin reductases . In: Biochimica et Biophysica Acta . 1698, 2004, pp. 1-26. doi : 10.1016 / j.bbapap.2003.10.003 .
- ↑ Flück CE et al. : P450 Oxidoreductase Deficiency - A New Form of Congenital Adrenal Hyperplasia . In: Endocr Dev. . 13, 2008, p. 67.