Methylene blue
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Methylene blue | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 16 H 18 ClN 3 S | |||||||||||||||||||||
| Brief description |
dark green, shiny crystals |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 319.86 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
approx. 190 ° C (decomposition) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Methylene blue (syn. Methylthioninium ) is a phenothiazine - derivative . The cationic dye is used in chemistry, medicine and dyeing technology.
As a pure dye, methylene blue appears as a dark green powder or as dark green crystals. Commercially available it is also available as a double salt with zinc chloride , which is a brown powder.
Historical
Methylene blue was first synthesized in 1876 by the chemist Heinrich Caro at BASF . A year later, BASF received the first German Reich patent for a tar dye for methylene blue . Around 1900, methylene blue was also tried as a drug for mental illness. It was not until the 1950s that other phenothiazines ( chlorpromazine ) were discovered as psychotropic drugs.
properties
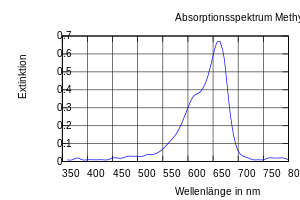
Methylene blue absorbs light in the range from about 530 to 700 nm; the absorption maximum is 660 nm.
Methylene blue is used as a redox indicator . It is a good hydrogen acceptor that oxidizes alcohols to aldehydes in the presence of platinum . The solution becomes discolored. An analogous reaction can also be carried out with glucose and atmospheric oxygen as a blue bottle experiment . A corresponding redox reaction takes place when diluted sulfuric acid and a little zinc powder are added to a methylene blue solution . Methylene blue is reduced to a colorless leuco form. The colorless solution turns blue again after shaking in air, because the leuco form is oxidized again to methylene blue by the oxygen in the air.
Manufacturing
Methylene blue is produced in several steps from N , N -dimethyl- p -phenylenediamine with the addition of dichromate as an oxidizing agent and the addition of N , N -dimethylaniline . Finally it has to be oxidized to the indamine, whereby Bindschedler's green is formed. This is cyclized with hydrogen sulfide in the presence of copper sulfate .
use
Colorants
It is used to dye fibers or paper blue. In water, methylene blue dissolves well with an intense blue color (hence the name), even small amounts cause a visible coloration of the water.
In histology , it was in 1885 for the first time by Paul Ehrlich certain selective staining tissues (especially the gray matter in the peripheral nervous system ) is used. Coloring with methylene blue is possible on the living organism ( vital coloring ), which is why it is one of the so-called vital coloring substances.
In molecular biology , methylene blue is used to stain DNA and RNA in gels and on membranes after blotting . Although methylene blue is not as sensitive as ethidium bromide , it is less toxic and it does not intercalate into the nucleic acid chains.
In botany it is next to ruthenium red for dyeing pectins used.
medicine
In medicine , it is an important antidote for nitrite and aniline poisoning, as it accelerates the conversion of methemoglobin back to functional hemoglobin (see methemoglobinemia ). It is also used as an antiseptic , to fight malaria , anti-rheumatic and for diagnostic purposes. In veterinary medicine, it is used together with malachite green as a remedy for the white spot disease that occurs in fish .
The versatile dye is also being investigated for its suitability for treating chronic lower back pain . The methylene blue is injected between the vertebrae directly into the damaged intervertebral discs (lat. Discus intervertebralis ), which destroys the pain receptors and thus eliminates or alleviates the pain. Preliminary results of the in a placebo -controlled clinical study conducted study are very encouraging: This simple, minimally invasive and inexpensive method of treatment results in a majority of patients to a sustainable, lasting for at least two years pain relief, with no patients complications occurred . Subsequent studies yielded partly contradicting results. The treatment method has not yet been approved .
Methylene blue is neurotoxic in certain concentrations . The use of methylene blue can lead to serotonin syndrome when using MAO inhibitors such as B. methylene blue and antidepressants of the group selective serotonin reuptake inhibitors (SSRI), such as fluoxetine , fluvoxamine , paroxetine , sertraline and citalopram can be combined.
In experimental pharmacology and already in clinical medicine, methylene blue is used as an enzyme inhibitor of soluble guanylyl cyclase and thus in catecholamine-refractory septic shock .
Others
In geology , the methylene blue method is used to determine the smectite content in clay minerals. It is an important quality control method in many branches of industry.
In analytical chemistry it is used in the determination of anionic surfactants according to the Longwell-Manience method and in the Epton titration .
In wastewater analysis , a methylene blue sample is used to determine the putrefactive properties. The methylene blue sample can be used to prove whether and to what extent the effluent of a sewage treatment plant still contains putrefactive substances. Methylene blue is a redox indicator and becomes discolored with absolute exclusion of air to the extent to which anaerobic conditions (H 2 S formation) take over. The time taken for the dye added to the sample to decolorize is determined. 0.6 ml of a methylene blue solution (0.05%) is added to a 100 ml ground-glass stopper bottle, filled to the brim with sample, the stopper is attached without bubbles and is kept in the dark at 20 ° C (incubator). The sample is observed daily (several times on the first day) and determines the time until discoloration; if this occurs on the first day (details in hours) or within four days (details in days), the process quality is not permitted; if no discoloration occurs within five days, the sample is designated as "ne" (not discolored) and the test is terminated (see DEV [1], H 22, "Test for putrefaction" and ÖNORM M 6276).
In biochemistry, methylene blue is used as a redox mediator. The half-value potential E 0 'is +0.011 mV.
literature
- Ulrich L. Bohne, Richard P. Kreher: Methylene blue. History of a dye - a dye with a history. In: NiU chemistry. No. 52, 1999, pp. 36-37.
Individual evidence
- ↑ a b c d e Entry on methylene blue. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ a b c d Entry on methylthioninium chloride in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on methylene blue in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Hans Bangen: History of the drug therapy of schizophrenia. Berlin 1992.
- ↑ Brigitte M. Gensthaler: Ehrlich's methylene blue - blue dye against malaria. In: Pharmaceutical newspaper. No. 39, 2004. HTML
- ↑ B. Peng et al .: A randomized placebo-controlled trial of intradiscal methylene blue injection for the treatment of chronic discogenic low back pain . In: Pain . tape 149 , no. 1 , 2010, p. 124-129 , PMID 20167430 .
- ↑ G. Gupta, M. Radhakrishna and a .: Methylene blue in the treatment of discogenic low back pain. In: Pain physician. Volume 15, Number 4, 2012 Jul-Aug, pp. 333-338, PMID 22828687 .
- ↑ JW Kallewaard, JW Geurts u. a .: Efficacy, Safety, and Predictors of Intradiscal Methylene Blue Injection for Discogenic Low Back Pain: Results of a Multicenter Prospective Clinical Series. In: Pain practice. Volume 16, number 4, April 2016, pp. 405-412, doi: 10.1111 / papr.12283 , PMID 25753429 .
- ↑ X. Zhang, J. Hao et al. a .: Clinical Evaluation and Magnetic Resonance Imaging Assessment of Intradiscal Methylene Blue Injection for the Treatment of Discogenic Low Back Pain. In: Pain physician. Volume 19, Number 8, 2016 Nov-Dec, pp. E1189-E1195, PMID 27906950 .
- ↑ HP Patel, DR Chadwick, BJ Harrison, SP Balasubramanian: Systematic review of intravenous methylene blue in parathyroid surgery. In: The British Journal of Surgery . Volume 99, number 10, October 2012, pp. 1345-1351, doi: 10.1002 / bjs.8814 , PMID 22961511 .
- ^ G. Sweet, SB Standiford: Methylene-blue-associated encephalopathy. In: Journal of the American College of Surgeons. Volume 204, number 3, March 2007, pp. 454-458, doi: 10.1016 / j.jamcollsurg.2006.12.030 , PMID 17324781 .
- ↑ Clonus, hyperreflexia, and agitation in a patient with high serum fluvoxamine levels: symptoms of serotonin toxicity. (PDF; 170 kB) In: Switzerland Med Forum. 8, 2008, pp. 100-103.
- ↑ psychotropical.com: Methylene Blue and Serotonin Toxicity: Introduction.
- ^ RR Ramsay, C. Dunford, PK Gillman: Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. In: Br J Pharmacol. 152 (6), 2007 Nov, pp. 946-951. PMID 17721552 .
- ↑ Entry on serotonin toxicity in the Pharmawiki , accessed on January 28, 2017.
- ↑ GM Tiboni, F. Giampietro, D. Lamonaca: The soluble guanylate cyclase inhibitor methylene blue evokes preterm delivery and fetal growth restriction in a mouse model. In: In Vivo. 2001 Jul-Aug; 15 (4), pp. 333-337. PMID 11695226 .
- ↑ Hans-Anton Adams: For diagnosis and therapy of shock forms. Recommendations of the DIVI Interdisciplinary Working Group on Shock - Part V Septic Shock. In: Anästh Intensivmed. 46, 2005, p. 290.
- ↑ MY Kirov, OV Evgenov, NV Evgenov, EM Egorina, MA Sovershaev, B. Sveinbjornsson, EV Nedashkovsky, LJ Bjertnaes: infusion of methylene blue in human Spetic shock: A pilot, randomized, controlled study. Crit Care Med 29, 2001, pp. 1860-1867.
- ↑ Hewitt, LF. "Oxidation-Reduction Potentials in Bacteriology and Biochemistry." Oxidation-Reduction Potentials in Bacteriology and Biochemistry. Edn 6 (1950).