Potassium peroxodisulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
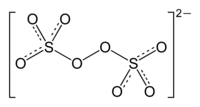
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Potassium peroxodisulfate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | K 2 S 2 O 8 | ||||||||||||||||||
| Brief description |
colorless and odorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 270.32 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.48 g cm −3 |
||||||||||||||||||
| Melting point |
Decomposition from ~ 100 ° C |
||||||||||||||||||
| boiling point |
decomposition |
||||||||||||||||||
| solubility |
Slightly soluble in water (50 g l −1 at 20 ° C), insoluble in alcohol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Potassium , often short potassium , often abbreviated as KPS , the potassium salt of peroxodisulfuric . The persulfate ion contains oxygen in the unstable oxidation state −1. That is why potassium persulfate is a radical generator and a very strong oxidizing agent or bleaching agent .
Extraction and presentation
Potassium peroxodisulphate is produced industrially by the electrolysis of concentrated potassium sulphate or potassium hydrogen sulphate solutions at high current densities (~ 1A / dm²). At the anode , sulfate ions are oxidized to peroxodisulfate ions , at the cathode water is reduced to hydrogen . The relatively poorly soluble potassium peroxodisulfate crystallizes out.
In the technical process for the anodic reaction platinum - or glassy carbon - electrodes that have a high overvoltage for oxygen evolution have used. Lead or graphite electrodes are used for the cathodic reaction . At low current densities and in dilute solutions, the sulfate radical anions formed as an intermediate product do not react with one another to form persulfate ion, but with water with the evolution of oxygen.
properties
If potassium peroxodisulfate solutions are heated (70–100 ° C) or exposed to UV radiation, the persulfate ion breaks down into sulfate radical anions. This reaction can be accelerated by suitable catalysts so that it takes place appreciably even at lower temperatures (20–50 ° C). Examples of catalysts are metallic platinum , silver (I) and copper (II) ions.
Persulfates are among the strongest oxidizing agents and their oxidizing power is only surpassed by fluorine , ozone and oxygen fluorides . However, the oxidation is relatively slow. They are able to oxidize almost all organic compounds. Iodine is slowly separated from iodide solutions , manganese (II) salt solutions are oxidized to manganese dioxide, in the presence of catalytically active silver (I) ions even up to permanganate .
Potassium peroxodisulfate is stable as a crystalline solid, but decomposes at higher temperatures with evolution of oxygen. At ~ 100 ° C the disintegration is complete. In a moist or impure state, it tends to decompose even at much lower temperatures. Therefore, the connection must be protected from direct sunlight and other heat sources during storage.
Solutions of potassium peroxodisulphate have an acidic reaction and disintegrate slowly at room temperature and quickly at elevated temperature. In a strongly acidic medium ( pH value <4) the decomposition takes place autocatalytically . The resulting acid catalyzes the further decomposition of the persulfate ions.
Potassium peroxodisulphate can react violently with reducing agents. Mixtures of potassium peroxodisulfate and flammable substances can burn in the absence of air.
use
Potassium peroxodisulfate is a widely used initiator for polymerisation in emulsion or in solution, for example in the production of polyacrylates , polyvinyl acetate and polyvinyl chloride and in the copolymerisation in emulsion of acrylonitrile , 1,3-butadiene , styrene and other monomers. These reactions with an amount of usually 0.1-0.5% potassium peroxodisulfate are usually carried out at 75 to 95 ° C. In combination with redox systems, polymerizations at lower temperatures are also possible. In cosmetics , potassium peroxodisulfate is used as an essential component of bleaching preparations for bleaching hair and as a bleaching component in hair dyes. Potassium peroxodisulfate is used in textile finishing as a desizing agent and as a bleach activator , especially for cold bleaching processes . It is also used as an oxidizing agent in chemical syntheses ( e.g. Elbs oxidation of phenols to p-diphenols in alkaline solution) and in analysis, for example as a disintegrating agent .
Potassium peroxodisulfate is also used in laboratories for cleaning glassware , in paper production for modifying starch , as a disinfectant and for the oxidative removal of pollutants (for example mercury in exhaust air treatment systems).
In the metal and electronics industry, potassium peroxodisulphate is used to a lesser extent for coloring brass black (method according to Erich Groschuff ) and for etching printed circuits ( circuit boards ). With the latter, either the surface is cleaned oxidatively (etching) or copper is removed oxidatively (etching). However, due to its higher solubility in water, sodium persulfate is preferred for this application.
In analog photography , potassium persulfate can be used as a reducer to remove traces of thiosulfate from negatives during film processing . In the past, potassium peroxodisulfate was also used as a flour treatment agent for "flour improvement". This has been banned in Germany since 1956/57.
Individual evidence
- ↑ Entry on POTASSIUM PERSULFATE in the CosIng database of the EU Commission, accessed on March 4, 2020.
- ↑ a b c d e f g h Entry on potassium peroxodisulphate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on potassium peroxodisulfate. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ Entry on Dipotassium peroxodisulphate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ G. Brauer (Ed.), Handbook of Preparative Inorganic Chemistry 2nd ed., Vol. 1, Academic Press 1963, pp. 392-3.
- ↑ Thomas Walter Jelinek: Practical Electroplating: A teaching and manual . Leuze, Bad Saulgau 2005, ISBN 3-87480-207-8 .
literature
- Pradyot Patnaik: Handbook of Inorganic Chemicals . McGraw-Hill, New York 2002 ISBN 0-07-049439-8 (English)








