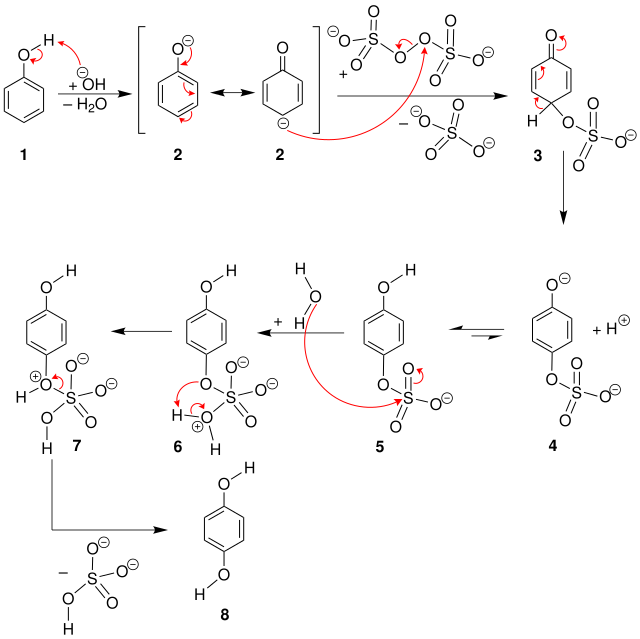Elbe oxidation
The Elbs oxidation (also called Elbs persulfate oxidation) is a name reaction of organic chemistry . The reaction was first published in 1893 by the German chemist Karl Elbs (1858–1933). It is used for the synthesis of hydroquinones from phenols . Salts of peroxodisulfuric acid such as potassium peroxodisulfate serve as oxidizing agents . The Elbs oxidation should not be confused with the Elbs reaction for the synthesis of polynuclear aromatics .
Reaction mechanism
The Elbs oxidation is carried out in a basic environment. First, phenol ( 1 ) is deprotonated to the mesomeric-stabilized phenolate ( 2 ) . The carbon atom in the para position then nucleophilically attacks the oxygen atom of the peroxodisulfate, creating an aromatic sulfate 5 . This is then hydrolyzed, thereby releasing the desired product 8 .
The para product is formed with good selectivity. Catechol or its derivatives are formed when the para position is already occupied by a substituent . However, the achievable yields are moderate.
Individual evidence
- ↑ K. Elbs: About Nitrohydroquinone. In: Journal for Practical Chemistry. 48, No. 1, 1893, pp. 179-185, doi : 10.1002 / prac.18930480123 .
- ^ Siegfried Hauptmann : Organic chemistry . 2nd reviewed edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, ISBN 3-342-00280-8 , p. 325.
- ↑ a b T. Laue, A. Plagens: Name and keyword reactions . 4th edition, Teubner, Wiesbaden 2004, ISBN 3-519-33526-3 , pp. 112-114.
- ^ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents. Volume 1, Wiley, 2009, ISBN 978-0-471-70450-8 (3-Volume Set), p. 977.
literature
- Suresh M. Sethna: The Elbs Persulfate Oxidation. In: Chemical Reviews. 49, No. 1, 1951, pp. 91-101, doi : 10.1021 / cr60152a002 .
- EJ Behrman: The Persulfate Oxidation of Phenols and Arylamines (The Elbs and the Boyland-Sims Oxidations) . In: Org. React. 35, 1988, ISBN 978-0-471-83253-9 , pp. 421-511.