Elbs reaction
The Elbs reaction is a name reaction of organic chemistry and named after the German chemist Karl Elbs . The reaction was first published in 1884 it is the pyrolysis of in ortho position methylated aromatic compound to form a fused aromatic system, for example. B. Anthracene :
mechanism
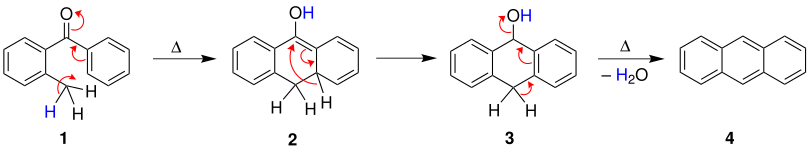
In principle, three mechanisms are possible for the Elbs reaction, with two useful mechanisms being described in this section. The first mechanism is proposed by Fieser and begins with a cyclization of the more methylated , aromatic acyl 1 under the action of heat. This is followed by a [1,3] -H shift, which results in compound 3 . After dehydration , the desired polyaromatic 4 is formed .
Cook describes the second mechanism. After a rearrangement under the action of heat and a cyclization , the compound 3 is formed . The desired polyaromatic 5 is then produced by a [1,3] -H shift and subsequent dehydration.
The acetyls required for the synthesis can be obtained via Friedel-Crafts acylation with aluminum chloride .
variants
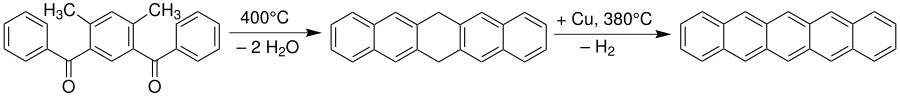
As shown in the previous sections, anthracene can be made accessible through dehydration. But larger aromatic systems, such as pentacene , can also be produced using an Elbs reaction. However, the reaction does not proceed here in one step, but leads to a dihydropentacene, which in a further step with copper as a catalyst dehydrated has to be.
Polynuclear heterocyclic compounds can also be synthesized by the Elbs reaction. In 1956, for example, the Elbs reaction of a derivative of thiophene was published. However, the expected linear system was not obtained. The reason for this lies in a changed reaction mechanism, which takes place over several radical steps after the formation of an intermediate product .
Individual evidence
- ^ A b Karl Elbs, Einar Larsen: Ueber Paraxylylphenylketon. In: Reports of the German Chemical Society. 17, No. 2, 1884, pp. 2847-2849, doi: 10.1002 / cber.188401702247 .
- ^ A b Karl Elbs: Contributions to the knowledge of aromatic ketones. First communication. In: Journal for Practical Chemistry. 33, No. 1, 1886, pp. 180-188, doi: 10.1002 / prac.18860330119 .
- ^ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents. Volume 1, Wiley, 2009, ISBN 978-0-471-70450-8 (3-Volume Set), p. 983.
- ↑ a b E. Breitmaier, G. Jung: Organic Chemistry . 5th edition, Thieme, Stuttgart 2005, ISBN 978-3-13-541505-5 , p. 183.
- ^ GM Badger, BJ Christie: Polynuclear heterocyclic systems. Part X. The elbs reaction with heterocyclic ketones. In: Journal of the Chemical Society (Resumed). 1956, pp. 3435-3437, doi: 10.1039 / JR9560003435 .