Barium carbide
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
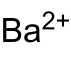 |
||||||||||
| General | ||||||||||
| Surname | Barium carbide | |||||||||
| other names |
Barium acetylide |
|||||||||
| Molecular formula | BaC 2 | |||||||||
| Brief description |
gray solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 161.35 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
3.74 g cm −3 |
|||||||||
| Melting point |
1065 ° C |
|||||||||
| solubility |
Decomposes in water and acids |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Barium carbide is a chemical compound of barium from the group of carbides . In addition to BaC 2 , at least one other barium carbide, BaC 6, is known.
Extraction and presentation
Barium carbide can be obtained by reacting barium with carbon at 1300 ° C.
The representation from barium sulphate with red phosphorus and carbon is also possible.
properties
Barium carbide belongs in the group of carbides to the acetylides, as it is formally derived from ethyne . It is a gray solid that reacts with water to give off ethyne.
Several modifications are known, the tetragonal shape being stable below 150 ° C. It crystallizes in the space group I 4 / mmm (space group no. 139) with the lattice parameters a = 439.4 pm and b = 712.5 pm. The Ba 2+ ion is coordinated by two carbon atoms of four neighboring C 2 2− ions and by one carbon atom of two further anions. At 769 ° C the tetragonal form changes into a cubic phase with the space group Fm 3 m (No. 225) , below −100 ° C a monoclinic phase with the space group C 2 / c (No. 15) forms .
Individual evidence
- ↑ a b c d e Dale L. Perry: Handbook of Inorganic Compounds, Second Edition . CRC Press, 2011, ISBN 978-1-4398-1461-1 , pp. 499 ( limited preview in Google Book search).
- ↑ a b c Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 978-0-444-59550-8 , pp. 358 ( limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ seilnacht.com: Seilnacht's Periodic Table: Barium , accessed on December 13, 2014
- ^ RK Sharma: Chemistry Of Hydrides And Carbides . Discovery Publishing House, 2007, ISBN 978-81-8356-227-0 , pp. 295 ( limited preview in Google Book search).
- ↑ a b V. Vohn, W. Kockelmann, U. Ruschewitz: On the synthesis and crystal structure of BaO 2 . In: Journal of Alloys and Compounds , 284, 1999, pp. 132-137, doi: 10.1016 / S0925-8388 (98) 00957-8 .

