Lithium chlorate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
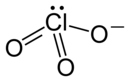
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Lithium chlorate | |||||||||||||||
| other names |
Lithium chlorate |
|||||||||||||||
| Molecular formula | LiClO 3 | |||||||||||||||
| Brief description |
colorless long hygroscopic needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 90.39 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.119 g cm −3 |
|||||||||||||||
| Melting point |
127.6-129 ° C |
|||||||||||||||
| boiling point |
270 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.64 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Lithium chlorate is the lithium salt of chloric acid and, like many chlorates, is a strong oxidizing agent at high temperatures .
Manufacturing
Lithium chlorate can be made from chloric acid and lithium carbonate .
The synthesis from barium chlorate and lithium sulfate has also been described.
properties
Lithium chlorate forms three different hydrates: a trihydrate LiClO 3 · 3 H 2 O, a monohydrate LiClO 3 · H 2 O and a quarter hydrate 4 LiClO 3 · H 2 O. The monohydrate changes into the quarter hydrate at 20.5 ° C transforms at 42 ° C in the anhydrate in order. This anhydrate crystallizes in the cubic crystal system .
At 270 ° C, lithium chlorate decomposes into lithium chloride and oxygen, and a side reaction is disproportionation to the next lower and the next higher oxidation level of chlorine .
use
Lithium chlorate is used as an oxidizing agent in rocket fuels .
Individual evidence
- ↑ a b c d e Jean D'Ans, Ellen Lax: Paperback for chemists and physicists. 3. Elements, inorganic compounds and materials, minerals, Volume 3. 4. Edition, Gabler Wissenschaftsverlage, 1997, ISBN 978-3-5406-0035-0 , p. 534 ( limited preview in the Google book search).
- ^ SS Wang, DN Bennion: The Electrochemistry of Molten Lithium Chlorate and Its Possible Use with Lithium in a Batter . In: J. Electrochem. Soc. 1983 , 130 (4), pp. 741-747. doi : 10.1149 / 1.2119796 .
- ↑ TO Campbell, EM Kartzmark, WB MaryK: The system Sodium Chlorate - Water - Dioxane and lithium chlorate - Water - dioxane, at 25 ° . In: Canadian Journal of Chemistry . 44, 1966, pp. 935-937, doi : 10.1139 / v66-136 .
- ↑ a b c d e R. Abegg, F. Auerbach, I. Koppel: Handbuch der inorganic Chemie . Verlag S. Hirzel, 1908, 2nd volume, 1st part, p. 136. Full text
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ AN Campbell, JE Griffiths: The System Lithium Chlorate - Lithium Chloride - Water at Various Temperatures . In: Canadian Journal of Chemistry . 34, 1956, pp. 1647-1661, doi : 10.1139 / v56-213 .
- ↑ E.-C. Koch: Special Materials in Pyrotechnics: III. Application of Lithium and its Compounds in Energetic Systems . In: Propellants, Explosives, Pyrotechnics 2004 , 29 (2). Pp. 67-80. doi : 10.1002 / prep.200400032



