Lithium chloride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Li + __ Cl - | ||||||||||
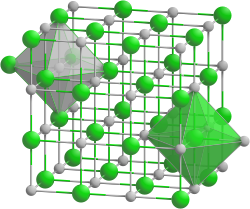
| Crystal system |
cubic |
|||||||||
| Space group |
Fm 3 m (No. 225) |
|||||||||
| Lattice parameters |
514 pm |
|||||||||
| Coordination numbers |
Li [6], Cl [6] |
|||||||||
| General | ||||||||||
| Surname | Lithium chloride | |||||||||
| Ratio formula | LiCl | |||||||||
| Brief description |
white solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 42.39 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
2.07 g cm −3 |
|||||||||
| Melting point |
614 ° C (anhydrous) |
|||||||||
| boiling point |
1360 ° C |
|||||||||
| solubility |
|
|||||||||
| Refractive index |
1.662 |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
Lithium chloride LiCl, the lithium salt of hydrochloric acid , forms colorless, highly hygroscopic crystals . In addition to the anhydrous lithium chloride, there are also various hydrates , known are LiCl · n H 2 O with n = 1, 3 and 5.
properties
Lithium chloride solutions are very hygroscopic . Anhydrous lithium chloride crystallizes out of concentrated aqueous solutions only at temperatures above 98 ° C. At lower temperatures one of the hydrate forms is obtained. The solubility in water is approx. 450 g LiCl / kg solution. Gaseous lithium chloride forms planar rings from several lithium chloride molecules (di-, tri- and oligomers).
Lithium chloride solutions are very corrosive. Suitable materials must be selected for handling concentrated solutions. Lithium chloride solutions also damage concrete .
The standard enthalpy of formation of the crystalline lithium chloride is Δ f H 0 298 = −408.27 kJ / mol.
Manufacturing
Lithium chloride is obtained by reacting an aqueous lithium hydroxide or lithium carbonate solution with hydrogen chloride and subsequent concentration and drying.
Technically relevant at the moment is only the conversion of lithium carbonate with hydrochloric acid with subsequent concentration with crystallization of lithium chloride in vacuum evaporators.
In addition, lithium chloride is often a by-product of organometallic syntheses (salt metathesis).
Since the synthesis from aqueous media always gives a compound containing water of crystallization under room conditions, the anhydrous salt is prepared by reacting the hydrate with thionyl chloride :
use
Lithium chloride can be used to make lithium . For this purpose, a mixture of lithium chloride and potassium chloride is used in a melt-flow electrolysis . Because of its strong hygroscopic effect, it can be used as a drying agent and also for dehumidifying rooms. It can also be used as a flux in soldering and welding technology. Due to its hygroscopicity, it can be used in dew point sensors or hygrometers. The electrical conductivity of the salt is strongly dependent on the water concentration, which is why the ambient humidity can be determined from the conductivity of the lithium chloride. Lithium chloride can be used as a tracer in chemical or geological investigations . Lithium chloride can be used in deicer solutions. However, since these are corrosive , they are banned for use on aircraft in the USA , for example . The textile industry also uses lithium chloride. Lithium chloride solutions with 25–30% LiCl can be used in cold baths. Such cold baths can remain liquid down to −70 ° C.
Lithium chloride is also suitable as a potential basis for a new treatment method against the varroa mite , a dangerous parasite of honeybees . However, it is fatal to open brood.
Web links
- B. Schwan / technology review: Solar heat in the absorber
Individual evidence
- ↑ a b c d e f Entry on lithium chloride in the GESTIS substance database of the IFA , accessed on February 22, 2017(JavaScript required) .
- ↑ a b c d data sheet lithium chloride (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ Dimethyl Sulfoxide (DMSO) Solubility Data. Gaylord Chemical Company, LLC; Bulletin 102, June 2014, p. 15. (PDF)
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Index of Refraction of Inorganic Crystals, pp. 10-246.
- ↑ A. Hönnerscheid, J. Nuss, C. Mühle, M. Jansen: The crystal structures of the monohydrates of lithium chloride and lithium bromide , in: Journal for inorganic and general chemistry 2003 , 629, 312-316.
- ↑ Dissertation: "Investigation of organic solid-state reactions using the example of substitution and polycondensation reactions", Oliver Herzberg, University of Hamburg 2000. Full text
- ^ Alfred R. Pray: Anhydrous metal chlorides . In: Therald Moeller (Ed.): Inorganic Syntheses . tape 5 . McGraw-Hill, Inc., 1957, pp. 153-156 (English).
- ↑ Jander, Blasius, Strähle: Introduction to the inorganic-chemical practical course . 14th edition. Hirzel, Stuttgart 1995, ISBN 978-3-7776-0672-9 , pp. 386-387.
- ↑ a b Script University of Duisburg-Essen (PDF; 268 kB)
- ↑ a b University of Karlsruhe script ( memento of the original from February 15, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 2.1 MB)
- ^ University of Colorado script
- ↑ Airport Winter Safety and Operations (PDF; 432 kB)
- ↑ Patent DE 19638319C1 1998
- ^ University of Hohenheim: Researchers discover a drug against Varroa mite. January 12, 2018, accessed January 14, 2018 .
- ↑ Bettina Ziegelmann, Elisabeth Abele, Stefan Hannus, Michaela Beitzinger, Stefan Berg: Lithium chloride effectively kills the honey bee parasite Varroa destructor by a systemic mode of action . In: Scientific Reports . tape 8 , no. 1 , January 12, 2018, ISSN 2045-2322 , doi : 10.1038 / s41598-017-19137-5 ( nature.com [accessed January 14, 2018]).
- ↑ Mellifera e. V .: Critical response to article about lithium chloride as an anti-varroa agent - Mellifera e. V. , accessed February 20, 2018



