Neptunium (III) fluoride
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ Np 3+ __ F - | |||||||
| Crystal system | |||||||
| Space group |
P 3 c 1 (No. 165) |
||||||
| Lattice parameters |
a = 712.9 pm |
||||||
| Coordination numbers |
Np [9], F [3] |
||||||
| General | |||||||
| Surname | Neptunium (III) fluoride | ||||||
| other names |
Neptunium trifluoride |
||||||
| Molecular formula | NpF 3 | ||||||
| Brief description |
purple solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 294.04 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
1425 ° C |
||||||
| Hazard and safety information | |||||||
 Radioactive |
|||||||
|
|||||||
| Thermodynamic properties | |||||||
| ΔH f 0 |
−360 kcal mol −1 |
||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Neptunium (III) fluoride is a chemical compound made up of the elements neptunium and fluorine . It has the formula NpF 3 and belongs to the fluoride class of substances .
presentation
Neptunium (III) fluoride is produced by reacting neptunium (IV) oxide (NpO 2 ) with hydrogen fluoride (HF) in an H 2 stream at 500 ° C.
It can also be produced by reacting an aqueous neptunium solution with fluoride salts in a weakly acidic environment .
Neptunium (IV) fluoride (NpF 4 ) is reduced to neptunium (III) fluoride in the H 2 flow.
properties
Physical Properties
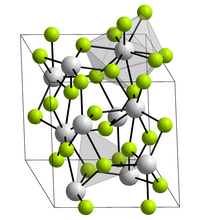
Neptunium (III) fluoride is a purple solid that melts at 1425 ° C. It crystallizes in the lanthanum fluoride structure with the lattice parameters a = 712.9 pm and c = 728.8 pm. Each neptunium nucleus is surrounded by nine fluorine nuclei in a distorted triple-capped trigonal-prismatic structure.
Chemical properties
Neptunium (III) fluoride is converted into volatile neptunium hexafluoride (NpF 6 ) in a flow of fluorine gas at 500 ° C :
The oxidation of neptunium (III) fluoride with an oxygen-hydrogen fluoride mixture leads to neptunium (IV) fluoride (NpF 4 ).
In an unusual reaction with oxygen (O 2 ), neptunium (IV) fluoride and neptunium dioxide are formed.
Metallic neptunium can be obtained from its compounds by reduction . First, neptunium (III) fluoride was reacted with elemental barium or lithium at 1200 ° C.
safety instructions
Classifications according to the CLP regulation are not available because they only include chemical hazard and play a completely subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
Individual evidence
- ↑ a b c C. Keller: The chemistry of Neptunium , in: Fortschr. chem. Forsch. , 1969/70 , 13/1 , p. 69.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1969.
- ↑ Neptunium (III) fluoride at www.webelements.com .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this substance has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ a b c Gmelin's Handbook of Inorganic Chemistry , System No. 71, Transurane, Part C, pp. 101-104.
- ↑ a b C. Keller: The chemistry of Neptunium , in: Fortschr. chem. Forsch. , 1969/70 , 13/1 , p. 67.
- ↑ John G. Malm, Bernard Weinstock, E. Eugene Weaver: The Preparation and Properties of NpF 6 ; a Comparison with PuF 6 , in: J. Phys. Chem. , 1958 , 62 (12), pp. 1506-1508 ( doi: 10.1021 / j150570a009 ).
- ^ Wissenschaft-Online-Lexika: Entry on “Neptuniumverbindungen” in the Lexikon der Chemie, accessed on April 7, 2010.
literature
- Zenko Yoshida, Stephen G. Johnson, Takaumi Kimura, John R. Krsul: Neptunium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006 ; ISBN 1-4020-3555-1 , pp. 699-812 ( doi : 10.1007 / 1-4020-3598-5_6 ).
- C. Keller: The chemistry of neptunium , in: Fortschr. chem. Forsch. , 1969/70 , 13/1 , pp. 1–124 ( doi: 10.1007 / BFb0051170 ).






