Titanium disilicide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
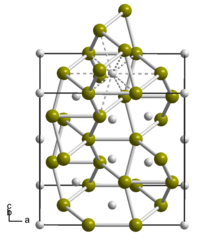
|
||||||||||||||||
| __ Ti __ Si | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Titanium disilicide | |||||||||||||||
| other names |
Titanium silicide (ambiguous) |
|||||||||||||||
| Ratio formula | TiSi 2 | |||||||||||||||
| Brief description |
white-gray to black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.07 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.02 g cm −3 |
|||||||||||||||
| Melting point |
1760 ° C |
|||||||||||||||
| solubility |
almost insoluble in water and mineral acids |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Titanium disilicide is an inorganic chemical compound of titanium from the group of silicides .
Extraction and presentation
Titanium disilicide can be obtained by reacting titanium or titanium hydride with silicon .
It is also possible to display it in an aluminothermic way by igniting a mixture of z. B. aluminum powder, sulfur , silicon dioxide and titanium dioxide or potassium fluorotitanate K 2 TiF 6 , by electrolysis of a melt potassium fluorotitanate and titanium dioxide or by reacting titanium with silicon tetrachloride .
It can also be obtained by reacting titanium (IV) chloride with monosilane , dichlorosilane or silicon.
properties
Titanium disilicide is a shiny metallic, white-gray to black crystalline solid with good thermal and electrical conductivity. It has an orthorhombic crystal structure with the space group Fddd (space group no. 70) (a = 825.3 pm , b = 478.3 pm, c = 854.0 pm). It is insoluble in water and mineral acids with the exception of hydrofluoric acid and slowly soluble in 10% potassium hydroxide solution. It should be noted that other titanium silicides are known in addition to titanium disilicide. For example, TiSi 1.8 with the space group Cmcm (No. 63) and a density of 3.9 gcm −3 , Ti 3 Si with a tetragonal crystal structure isotype to that of Ti 3 P, Ti 5 Si 4 with a melting temperature of 2120 ° C and a tetragonal crystal structure isotype to that of Zr 5 Si 4 and titanium monosilicide TiSi with a melting point of 1760 ° C and an orthorhombic crystal structure, isotype to that of iron boride FeB. A Ti 5 Si 3 compound is also known.
use
Titanium disilicide is used in the semiconductor industry (e.g. in the salicide process ). It can be used to split water with the help of sunlight.
Individual evidence
- ↑ a b c d e f Georg Brauer (Hrsg.): Handbook of Preparative Inorganic Chemistry . 3., reworked. Edition. tape II . Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1389 .
- ↑ a b c d e f g h data sheet Titanium silicide, 99.5% (metals basis) from AlfaAesar, accessed on June 15, 2013 ( PDF )(JavaScript required) .
- ^ Hugh O. Pierson: Handbook of Chemical Vapor Deposition, 2nd Edition: Principles, Technology ... William Andrew, 1999, ISBN 0-8155-1743-2 , pp. 331 ( limited preview in Google Book search).
- ↑ Data sheet Titanium silicide Ti 5 Si 3 , 99.5% (metals basis) from AlfaAesar, accessed on June 15, 2013 ( PDF )(JavaScript required) .
- ↑ a b Lih J. Chen: Silicide Technology for Integrated Circuits (Processing) . IET, 2004, ISBN 0-86341-352-8 , pp. 49 ff . ( limited preview in Google Book search).
- ↑ pro-physik.de: Splitting water with sunlight , September 26, 2007.
- ↑ Peter Ritterskamp, Andriy Kuklya, Marc-Andre Wüstkamp, Klaus Kerpen, Claudia Weidenthaler, Martin Demuth: A semiconducting catalyst based on titanium disilicide for splitting water with sunlight - reversible storage of oxygen and hydrogen. In: Angewandte Chemie. 119, 2007, pp. 7917-7921, doi: 10.1002 / anie.200701626 .





