Tungsten (V) fluoride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
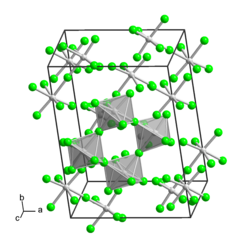
|
||||||||||
| __ W 5+ __ F - | ||||||||||
| General | ||||||||||
| Surname | Tungsten (V) fluoride | |||||||||
| other names |
Tungsten tafluoride |
|||||||||
| Ratio formula | WF 5 | |||||||||
| Brief description |
yellow solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 278.83 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
20 ° C (decomposition) |
|||||||||
| solubility |
Decomposes in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Tungsten (V) fluoride is a chemical compound of tungsten from the group of fluorides .
Extraction and presentation
Tungsten (V) fluoride can be obtained by reducing tungsten (VI) fluoride with tungsten .
The representation from the elements is also possible.
properties
Tungsten (V) fluoride is a yellow solid that decomposes in water. In solid form, like niobium (V) fluoride and tantalum (V) fluoride, the compound has a tetrameric structure.
Individual evidence
- ↑ a b c d e William M. Haynes: CRC Handbook of Chemistry and Physics, 94th Edition . CRC Press, 2016, ISBN 978-1-4665-7115-0 , pp. 97 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Kirk-Othmer Encyclopedia of Chemical Technology - Kirk-Othmer . Wiley, 2007, 2007, ISBN 978-0-471-48497-4 , pp. 378 ( limited preview in Google Book search).
- ^ A b Egon Wiberg, Nils Wiberg: Inorganic Chemistry . Academic Press, 2001, ISBN 978-0-12-352651-9 , pp. 1394 ( limited preview in Google Book Search).
- ^ Catherine E. Housecroft, AG Sharpe: Inorganic Chemistry . Pearson Education, 2005, ISBN 978-0-13-039913-7 , pp. 662 ( limited preview in Google Book search).
