Sodium telluride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
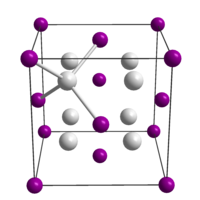
|
||||||||||||||||
| __ Na + __ Te 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium telluride | |||||||||||||||
| other names |
Disodium telluride |
|||||||||||||||
| Ratio formula | Na 2 te | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 173.58 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.90 g cm −3 |
|||||||||||||||
| Melting point |
953 ° C |
|||||||||||||||
| solubility |
Decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium telluride is an inorganic chemical compound of sodium from the group of tellurides .
Extraction and presentation
Sodium telluride can be obtained by reacting sodium with tellurium in liquid ammonia in the absence of air and moisture.
properties
Sodium telluride is a moisture-sensitive, white, odorless solid that immediately decomposes in air, giving it a dark color. It is soluble in water, whereby the solution quickly deposits black, powdery tellurium when exposed to air. It crystallizes in the cubic anti-calcium fluoride type with the space group Fm 3 m (space group no. 225) .
use
Sodium telluride can be used in organic syntheses.
Individual evidence
- ↑ a b c d e f g Data sheet Sodium telluride, 99.9% (metals basis) from AlfaAesar, accessed on December 5, 2013 ( PDF )(JavaScript required) .
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 431.
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 628 ( limited preview in Google Book search).
- ^ Edward W. Abel, FGA Stone, Alexander McKillop, Geoffrey Wilkinson: Main-Group Metal Organometallics in Organic Synthesis . Elsevier, 1995, ISBN 0-08-042318-3 , pp. 577 ( limited preview in Google Book search).


