Silicon disulfide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| __ Si 4+ __ S 2− | ||||||||||||||||
| Space group |
Ibam (No. 72) |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Silicon disulfide | |||||||||||||||
| other names |
Silicon (IV) sulfide |
|||||||||||||||
| Ratio formula | SiS 2 | |||||||||||||||
| Brief description |
white solid with a rotten egg smell |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 92.21 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.02 g cm −3 |
|||||||||||||||
| Melting point |
1090 ° C (sublimation) |
|||||||||||||||
| solubility |
Decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Silicon disulfide is an inorganic chemical compound of silicon from the group of sulfides .
Occurrence
It is believed that silicon disulfide occurs in circumstellar shells.
Extraction and presentation
Silicon disulfide can be obtained by reacting silicon dioxide with aluminum sulfide at 1200 to 1300 ° C., silicon monosulfide also being formed.
The preparation by reaction of dry hydrogen sulfide with silicon at 1200 to 1300 ° C or the thermal decomposition of tetraethyl mercaptosilane Si (SC 2 H 5 ) 4 at 250 to 300 ° C is also possible. It is also formed when the elements melt together at 1000 ° C.
properties
Silicon disulfide is a white, fibrous mass that is very sensitive to moisture and decomposes in water, ethanol and ammonia.
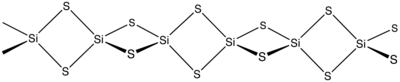
It burns slowly when heated in air. Unlike silicon dioxide, it does not have a spatial network, but a fiber structure with distorted tetrahedrally coordinated silicon atoms. It is a distorted cubic closest packing of spheres in which a quarter of the tetrahedral holes are occupied by silicon atoms. It is an orthorhombic crystal structure with the space group Ibam (space group no. 72) . When heated under pressure, this changes into a cristobalite- like modification.
Individual evidence
- ↑ a b c d e f g Data sheet Silicon (IV) sulfide, 95% from AlfaAesar, accessed on January 6, 2014 ( PDF )(JavaScript required) .
- ^ Goebel, JH (1993): SiS 2 in Circumstellar Shells . Astronomy and Astrophysics 278 (1): 226-230. bibcode : 1993A & A ... 278..226G .
- ↑ a b c d Georg Brauer , with the assistance of Marianne Baudler a . a. (Ed.): Handbook of Preparative Inorganic Chemistry . 3rd, revised edition. tape I . Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 699 .
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 916.
- ↑ by Will Kleber , Hans-Joachim Bautsch , Joachim Bohm : Introduction to crystallography - Will Kleber, Hans-Joachim Bautsch, Joachim Bohm . Oldenbourg Verlag, 2010, ISBN 3-486-59885-6 , p. 157 ( limited preview in Google Book search).
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 734 ( limited preview in Google Book search).