Aluminum nitrate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
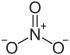
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aluminum nitrate | ||||||||||||||||||
| Molecular formula | Al (NO 3 ) 3 | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | |||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
Decomposition from 150 ° C |
||||||||||||||||||
| solubility | |||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aluminum nitrate is a chemical compound , the aluminum salt of nitric acid . It forms colorless and deliquescent rhombic crystals. Aluminum nitrate is very soluble in water; the solution is acidic. When crystallizing from aqueous solutions, the nona hydrate Al (NO 3 ) 3 · 9 H 2 O is formed. When heated, the hydrate gives off the water of crystallization at 73 ° C. The salt does not have a melting point and decomposes from around 150 ° C.
presentation
The representation of aluminum nitrate can be done by dissolving aluminum hydroxide in nitric acid.
use
The fabric was formerly the mantle used -Fabrication. In the manufacture of nuclear fuel rods , the nitrate is used as an extractant for uranium. Furthermore, aluminum nitrate is used as a mordant in dyeing and tanning of leather used.
safety instructions
Aluminum nitrate has a strong irritant effect on the mucous membranes of the eyes and the respiratory tract. In the case of oral intake, the ingested nitrate ions can lead to dizziness, headaches and pain in the abdominal area, bloody vomiting, diarrhea, slackening of the vascular muscles and a reduction in heart rate. The nitrate is a strong oxidizing agent and therefore has a fire-promoting effect.
Individual evidence
- ↑ a b c d Entry on aluminum nitrate. In: Römpp Online . Georg Thieme Verlag, accessed on April 7, 2014.
- ↑ a b c d e f g Entry on aluminum nitrate in the GESTIS substance database of the IFA , accessed on February 17, 2017(JavaScript required) .

![{\ displaystyle \ mathrm {\ \! \ {\ Biggr]} _ {3} ^ {-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9db8459a48d964bf7f88fb4159c82f09c14fe41d)



