Zirconium (III) iodide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ Zr 3+ __ I - | |||||||
| General | |||||||
| Surname | Zirconium (III) iodide | ||||||
| other names |
Zirconium triiodide |
||||||
| Ratio formula | ZrI 3 | ||||||
| Brief description |
dark blue solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 471.93 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
5.18 g cm −3 |
||||||
| Melting point |
275 ° C (decomposition) |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Zirconium (III) iodide is an inorganic chemical compound of zirconium from the group of iodides .
Extraction and presentation
Zirconium (III) iodide can be obtained by reacting zirconium (IV) iodide with zirconium at 500-700 ° C.
It is also possible to use aluminum instead of zirconium.
Gaseous zirconium (III) iodide can be obtained by reacting zirconium with hydrogen iodide at 1100–1500 K.
properties
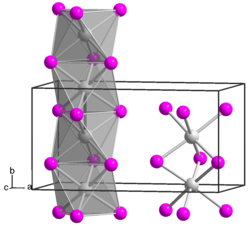
Zirconium (III) iodide is a blue solid. From a temperature of 275 ° C a disproportionation to zirconium (II) iodide and zirconium (IV) iodide takes place. The compound has an orthorhombic crystal structure with the space group Pmmn (space group no. 59) and a unit cell with the lattice constants a = 1295.4 pm, b = 667.9 pm and c = 729.2 pm. The ions form a one-dimensional chain structure, with two [ZrI 6/2 ] octahedra sharing two opposite triangular faces.
Individual evidence
- ↑ a b c d Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists . Springer DE, 1997, ISBN 3-540-60035-3 , pp. 820 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ FR Sale, RAJ Shelton: Studies in the chemical metallurgy of the titanium group metals. In: Journal of the Less Common Metals . 9, 1965, pp. 60-63, doi: 10.1016 / 0022-5088 (65) 90036-6 .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 916.
- ^ Paul L. Brown: Chemical Thermodynamics of Zirconium . Gulf Professional Publishing, 2005, ISBN 0-444-51803-7 , pp. 320 ( limited preview in Google Book search).
- ^ Abdessadek Lachgar, Douglas S. Dudis, John D. Corbett: Revision of the structure of zirconium triiodide. The presence of metal dimers. In: Inorganic Chemistry . 29, 1990, pp. 2242-2246, doi: 10.1021 / ic00337a013 .
- ↑ Ralf Alsfasser, HJ Meyer: Moderne Anorganische Chemie . Walter de Gruyter, 2007, ISBN 3-11-019060-5 , p. 350 ( limited preview in Google Book Search).

