Strontium sulfide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
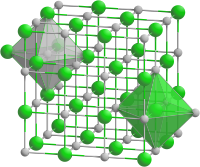
|
||||||||||||||||
| __ Sr 2+ __ S 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Strontium sulfide | |||||||||||||||
| other names |
Strontium monosulfide |
|||||||||||||||
| Ratio formula | SrS | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 119.69 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.65 g cm −3 |
|||||||||||||||
| Melting point |
2226 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Strontium sulfide is an inorganic chemical compound of strontium from the group of sulfides .
Extraction and presentation
Strontium sulfide can be obtained by reacting strontium sulfate with carbon.
Strontium sulfide of high purity can very easily be obtained in small quantities by heating strontium carbonate at about 1000 ° C in a sufficiently strong stream of hydrogen sulfide and hydrogen .
Production directly from the elements in an inert atmosphere is also possible.
properties
Strontium sulfide is a white solid that oxidizes in air and decomposes when damp. It has a crystal structure of the sodium chloride type (a = 6.020) with the space group Fm 3 m (space group no. 225) . It decomposes in acids with the formation of hydrogen sulfide.
use
Strontium sulfide is used for afterglow colors (blue-green light) and for hair removal.
Individual evidence
- ↑ a b c d Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 927.
- ↑ a b c data sheet Strontium sulfide, ≥99.9% from Sigma-Aldrich , accessed on March 25, 2013 ( PDF ).
- ↑ a b c W. M. Haynes, David R. Lide, Thomas J. Bruno: CRC Handbook of Chemistry and Physics 2012-2013 . CRC Press, 2012, ISBN 1-4398-8049-2 , pp. 4–92 ( limited preview in Google Book Search).
- ↑ a b c Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 140 ( limited preview in Google Book search).
- ↑ by Karl-Heinz Lautenschläger, Werner Schröter: Taschenbuch der Chemie - Karl-Heinz Lautenschläger, Werner Schröter . Harri Deutsch Verlag, 2008, ISBN 978-3-8171-1761-1 , pp. 883 ( limited preview in Google Book Search).
- ^ Robert E. Krebs: The History And Use of Our Earth's Chemical Elements: A Reference Guide . Greenwood Publishing Group, 2006, ISBN 0-313-33438-2 , pp. 78 ( limited preview in Google Book search).



