Potassium phosphide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ K + __ P 3− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium phosphide | |||||||||||||||
| other names |
Tripotassium phosphide |
|||||||||||||||
| Ratio formula | K 3 P | |||||||||||||||
| Brief description |
green solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 148.27 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.75 g cm −3 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium phosphide is an inorganic chemical compound of potassium from the group of phosphides .
Extraction and presentation
Potassium phosphide can be obtained by reacting potassium with phosphorus under argon at 200 ° C.
properties
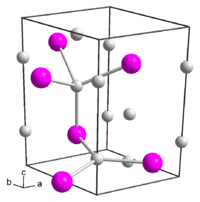
Potassium phosphide is an extremely air-sensitive green solid with a hexagonal crystal structure ( space group P 6 3 / mmc (space group no. 194) , lattice parameters a = 5.691 Å and b = 10.05 Å). It reacts violently with water. The structure contains two crystallographically different potassium ions. The first is trigonal-planar surrounded by three phosphide ions, the second tetrahedral .
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 960.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ G. Gnutzmann, FW Dorn, W. Klemm: The behavior of alkali metals to semimetals. VII. About some A 3 B and AB 2 compounds of the heavy alkali metals with elements of the V group. In: Journal for inorganic and general chemistry , 309, 1961, pp. 210-225, doi: 10.1002 / zaac.19613090308 .
- ↑ NOAA: POTASSIUM PHOSPHIDE | CAMEO Chemicals , accessed April 10, 2014.
