Cesium chlorate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
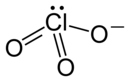
|
||||||||||||||||
| Crystal system |
trigonal |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Cesium chlorate | |||||||||||||||
| Molecular formula | CsClO 3 | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 216.36 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.57 g cm −3 |
|||||||||||||||
| Melting point |
226-228 ° C |
|||||||||||||||
| solubility |
soluble in water (63 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Cesium chlorate is an inorganic chemical compound with the ratio formula CsClO 3 .
properties
Cesium chlorate is a colorless solid which has a trigonal crystal structure with the space group R 3 m (space group no. 160) . The salt is stable up to its melting point and a little beyond it and is therefore a very stable chlorate . It decomposes at high temperatures in two parallel ways:
Since cesium perchlorate is still stable at temperatures around the melting point of cesium chlorate, it accumulates together with cesium chloride during decomposition .
Individual evidence
- ^ A b Jean d 'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 410 ( limited preview in Google Book search).
- ^ A b c d e Chemical Dictionary Online: Cesium chlorate .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Kurt H. Stern: High Temperature Properties and Thermal Decomposition of Inorganic Salts ... CRC Press, 2000, ISBN 1-4200-4234-3 , pp. 195 ( limited preview in Google Book search).

