Calcium hydroxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| __ Ca 2+ __ OH - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Calcium hydroxide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | Ca (OH) 2 | ||||||||||||||||||
| Brief description |
colorless, odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 74.10 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.24 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
Decomposes at 550 ° C |
||||||||||||||||||
| solubility |
poor in water (1.7 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 5 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Calcium hydroxide (also: hydrated lime , slaked lime , (white) hydrated lime , hydrated lime ) is the hydroxide of calcium .
Occurrence
Calcium hydroxide also occurs naturally as the mineral portlandite .
Extraction and presentation
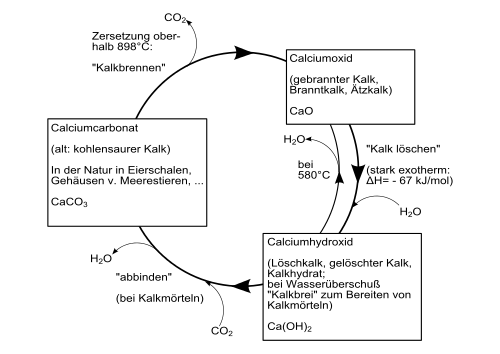
Calcium hydroxide is formed with strong heat development ( exothermic reaction ) when calcium oxide is mixed with water. This process is also called lime drying . The heat development is so strong that some of the water also evaporates (colloquially known as "smoking").
The following diagram gives an overview of the conversion processes between different calcium compounds ( technical lime cycle ) :
It is also possible to display it by reacting aqueous calcium salt solutions with alkaline solutions (for example calcium nitrate with potassium hydroxide ).
Calcium hydride or calcium itself reacts violently with water to form calcium hydroxide and hydrogen .
properties
Calcium hydroxide is a colorless powder that only slightly dissolves in water. The solubility depends on the temperature and decreases with increasing temperature: 1860 mg / l at 0 ° C; 1650 mg / l at 20 ° C and 770 mg / l at 100 ° C. At 580 ° C it decomposes, producing calcium oxide and water. Calcium hydroxide consists of trigonal crystals with the polytype 2H of the crystal structure of the cadmium iodide type ( space group P 3 m 1 (space group No. 164) , a = 3.584, c = 4.896 Å ).
Calcium hydroxide has a strongly basic reaction, although it is not very soluble : The pH value of a saturated solution is 11–12.6.
use
The main use of calcium hydroxide is the preparation of mortar in construction. It is used there under the name of white lime hydrate (DIN 1060). Lime plasters consist of mixtures of calcium hydroxide and sand. The latter can also be added in the form of ground limestone . Asphalt mix is also increasingly being added to improve the durability of the finished asphalt layer.
Calcium hydroxide is formed when Portland cement hardens. Portland cement is mainly used to manufacture reinforced concrete . The alkaline effect of calcium hydroxide in concrete prevents the reinforcing steel from rusting until it is neutralized by carbonic acid (or other acidic components of rainwater, for example).
The antiseptic , caustic effect, which hinders the growth of pathogens and mold , is the reason why slaked lime was previously used to disinfect stables (the "lime" of stables).
In conjunction with soda and lubricating soap , slaked shell limestone is processed into Tadelakt , a hydrophobic lime plaster for wet rooms .
Lime is used to improve the load-bearing capacity of the building ground. A soil with too high a water content and the resulting low load-bearing capacity and poor compactability can be improved by mixing in 2-4% MA lime. The lime binds part of the water and thus improves, among other things, the plasticity, the compressibility and the load-bearing capacity. That is why soil improvement with lime is a method for immediately achievable improvement in the ability to install and facilitate the execution of construction work.
Slaked lime is used as an alternative to limestone in flue gas desulphurisation , as it forms calcium sulphate (gypsum) with sulfuric acid . The amount used is around 1.8 times less than for limestone. The resulting plaster has a whiteness of 80% and can be used commercially. Due to its high reactivity, lower consumption quantities are required. The disadvantage is the higher price compared to limestone.
In the food industry it is added to foods as an acidity regulator and is generally approved in the EU as a food additive with the designation E 526 without maximum quantity restriction ( quantum satis ) for food.
It is also used as a drug in dentistry , primarily for disinfecting root canals and cavities and for stimulating new dentin formation.
It is part of the soda lime , which is used in anesthesia machines or diving machines with rebreathing to eliminate carbon dioxide from the exhaled air.
Calcium hydroxide is also used as a pesticide in fruit growing . Here it is used, for example, as a fungicide (an agent against fungal infestation, such as tree cancer ).
Lime water is the (almost) saturated solution of calcium hydroxide and serves as a clear liquid for the detection of carbon dioxide through the formation of calcium carbonate, which precipitates and makes the solution cloudy.
Suspensions in water are:
- Fatty lime ( sump lime ): a creamy, stiff mass - building material: lime mortar
- Lime milk : a whitish, milk-like liquid that separates into sump lime and lime water - lime color , neutralization of acids, decarbonisation , flue gas desulphurisation
Calcium hydroxide is used as an intermediate product in the production of chlorinated lime and caustic soda from soda .
safety instructions
Burnt (unslaked) lime ( calcium oxide , quicklime) and slaked lime are irritating, contact with the eyes can cause serious eye damage. An aqueous calcium hydroxide solution is alkaline and slightly corrosive. Unslaked lime can cause fires when exposed to water due to the generation of heat.
Historical
The exothermic reaction of the slaked lime was considered one of the greatest everyday puzzles from antiquity to the early modern period and found a wide variety of explanations: While the church father Augustine of Hippo (354-430) in his " God's state " (21, 4) the phenomenon as looks at a kind of proof of God , the natural philosophers tried to interpret the phenomenon according to their respective ideas. Prominent examples are:
Seneca the Younger and the Stoa
According to the Stoic cosmology , the Roman philosopher Seneca the Younger († 65 AD) interpreted the lime slaughtering according to the four-element theory of Aristotle . The calcium is to him after firing a "fiery stone", which the heat of that makes him penetrating water.
Vitruvius and ancient building materials
In the second book of his “Ten Books on Architecture”, written around 30 BC, the ancient master builder Vitruvius formulated a building material science corresponding to the understanding of his time . There he tries in the 2nd and 5th sections to give a conclusive explanation of lime slaking. To this end, he combines the Greek atomism of Democritus and Epicurus with the geometric matter models of Pythagoras to create a completely separate theory of matter: For Vitruvius, the world consists of both " atoms " and vacuum (according to Democritus / Epicurus) and four elements, which however (according to Pythagoras) are geometric solids. Therefore, for him the “atoms” are identical with the Pythagorean bodies and move in empty space. When lime is burned, the "water and air atoms" leave the limestone, which is thought of as a lattice structure made of Pythagorean bodies , so that "holes" are created. Vitruvius explains the weight loss while burning. “Fire atoms”, on the other hand, are stored. When extinguishing, "water atoms" penetrate through the "holes" in the lime lump and the stored "fire" escapes. Since the material itself remains unchanged, the sand adheres to the mortar solely through the pores created in this way. While this explanation was sufficient for pure construction practice, the incompatibility of the systems combined by Vitruvius - atomistics does not recognize any geometrically different, modular "atomic bodies" - especially the architectural theorists of the Renaissance, who referred to him, such as Cesare Cesariano and Daniele Barbaro , posed additional puzzles .
Scaliger and Cardano
In the 16th century, two of the greatest polymaths of their time, Julius Caesar Scaliger and Gerolamo Cardano , waged a feud over the exact nature of the world over several years and book publications. While Gerolamo Cardano viewed matter as an infinite continuum, which only varied in its “density” from place to place, Scaliger took the view that the “ vacuum ” was the determining factor for the cosmos and the cause of all movement. In his main work, De subtilitate ad Cardanum (1557), directed against the adversary , Scaliger introduces this understanding of matter ( Exercitatio, 5,8-9 ) using nothing other than the "riddle of the slaked lime": Since the lump of lime absorbs water when it is slaked, its pores can only contain “vacuum”, since “air” cannot escape upwards in front of it, as it would immediately meet other “air” and would be blocked.
Individual evidence
- ↑ Entry on CALCIUM HYDROXIDE in the CosIng database of the EU Commission, accessed on February 24, 2020.
- ↑ Entry on E 526: Calcium hydroxide in the European database for food additives, accessed on June 27, 2020.
- ↑ a b c d e data sheet calcium hydroxide (PDF) from Merck , accessed on April 4, 2012.
- ↑ a b c d e Entry on calcium hydroxide in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 1305-62-0 or calcium hydroxide ), accessed on November 2, 2015.
- ↑ a b c Georg Brauer (ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II. Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 926.
- ↑ Roland Pfestorf, Heinz Kadner: Chemistry: A textbook for colleges. ISBN 978-3-81711783-3 , p. 368.
- ↑ Paperback Chemical Substances , 3rd edition. Harri Deutsch, Frankfurt a. M., 2007.
- ↑ Dr-Luthardt.de: pH value and solubility product of calcium hydroxide , accessed on November 21, 2019.
- ^ Article from asphalt 4/2010 on schaeferkalk.de: Lime hydrate in asphalt. , Retrieved February 28, 2017.
- ↑ Felix Henke / Laura Thiemann, Vitruvus on stucco and plaster - the relevant passages of the 'decem libri de architectura' , in: Firmitas et Splendor. Vitruvius and the techniques of wall decor , ed. by Erwin Emmerling, Andreas Grüner et al., Munich 2014 (studies from the Chair of Restoration, Technical University of Munich , Faculty of Architecture) ISBN 978-3-935643-62-7 , pp. 13–125, there p. 55.
- ↑ Thomas Reiser, The lime-scale according to ancient and rinascimental theories of matter. Notes on Vitruvius 2, 2 and 2, 5. By Cesariano and Barbaro on the feud between Scaliger and Cardano , in: Firmitas et Splendor (2014), pp. 299–319, there pp. 303–304.
- ↑ Henke / Thiemann (2014), pp. 57–59; Reiser (2014), pp. 306-312.
- ↑ Reiser (2014), pp. 314-317.