Potassium hydrogen sulfite
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
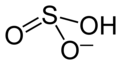
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Potassium hydrogen sulfite | ||||||||||||||||||
| other names |
Potassium bisulfite |
||||||||||||||||||
| Molecular formula | KHSO 3 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 120.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
190 ° C (decomposition) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Potassium hydrogen sulfite is a potassium salt of sulphurous acid and has the empirical formula KHSO 3 .
Extraction and presentation
Potassium hydrogen sulfite can be obtained by reacting sulfur dioxide with a potassium carbonate solution.
use
Potassium hydrogen sulfite is z. B. Wine added as an antioxidant and preservative . The permissible concentration is based on the statutory maximum quantities of sulfur dioxide. In addition, potassium hydrogen sulfite is used in a variety of other foods, such as starch, sago, pearl barley, dried potato products, vegetables, dried fruits, nuts, meat and fish.
It is approved in the EU as a food additive with the number E 228 .
The compound gives off sulfur dioxide.
Side effects
Excessive consumption of potassium hydrogen sulfite can lead to headaches , migraines and nausea . In addition, B vitamins and folic acid are destroyed. Food with this addition must have the number E228 in the list of ingredients .
Individual evidence
- ↑ a b c d e William M. Haynes: CRC Handbook of Chemistry and Physics, 97th Edition . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 99 ( limited preview in Google Book search).
- ↑ There is not yet a harmonized classification for this substance . A labeling of potassium hydrogen sulphite in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on March 4, 2020, is reproduced from a self-classification by the distributor .
- ^ Entry on Potassium Hydrogen Sulphite in the Hazardous Substances Data Bank , accessed April 16, 2018.
- ↑ George A. Burdock: Encyclopedia of Food and Color Additives . CRC Press, 1997, ISBN 978-0-8493-9412-6 , pp. 3000 ( limited preview in Google Book Search).
