Palladium (II) iodide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
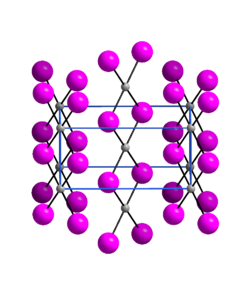
|
||||||||||||||||
| __ Pd 2+ __ I - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Palladium (II) iodide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | PdI 2 | |||||||||||||||
| Brief description |
black solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 360.23 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
6.003 g cm −3 |
|||||||||||||||
| Melting point |
350 ° C (decomposition) |
|||||||||||||||
| solubility |
slightly soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Palladium (II) iodide is an inorganic chemical compound of palladium from the group of iodides .
Extraction and presentation
Palladium (II) iodide can be obtained by reacting a very dilute solution of palladium in nitric acid with sodium iodide at 80 ° C.
The high-temperature modification α-palladium (II) iodide can be produced by reacting the elements at a reaction temperature above 600 ° C. The γ-modification is produced as a finely crystalline, almost X-ray amorphous powder through the precipitation of palladium (II) compounds with iodine salts from aqueous H 2 PdCl 4 solution at room temperature. When this modification is heated in dilute hydrogen iodide solution, it is converted into the β phase from approx. 140 ° C.
properties
Palladium (II) iodide is an almost X-ray amorphous, black powder. It is insoluble in acids but soluble in a potassium iodide solution. The compound comes in three modifications. The α-modification has an orthorhombic crystal structure with the space group Pnmn (space group no. 58, position 5) .
Individual evidence
- ↑ a b c d e f g data sheet Palladium (II) iodide, Premion®, 99.998% (metals basis), Pd 29% min from AlfaAesar, accessed on August 31, 2013 ( PDF )(JavaScript required) .
- ↑ a b Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 17.
- ↑ Kristin Brendel: Binary and ternary compounds of the platinum metals palladium and rhodium with tellurium and halogens. Preparations and structural characterization , dissertation, University of Freiburg 2001, urn : nbn: de: bsz: 25-opus-1971 .
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 668 ( limited preview in Google Book search).
