Ruthenium (III) bromide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
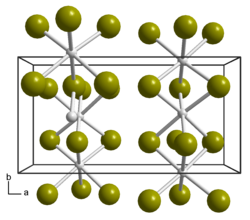
|
|||||||||||||||||||
| __ Ru 3+ __ Br - | |||||||||||||||||||
| Crystal system |
orthorhombic |
||||||||||||||||||
| Space group |
Pmmn (No. 59) |
||||||||||||||||||
| Lattice parameters |
a = 1126 pm, b = 587 pm, c = 650 pm |
||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ruthenium (III) bromide | ||||||||||||||||||
| other names |
Ruthenium tribromide |
||||||||||||||||||
| Ratio formula | RuBr 3 | ||||||||||||||||||
| Brief description |
black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 340.78 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
5.3 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
500 ° C (decomposition) |
||||||||||||||||||
| solubility |
almost insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−184 kJ mol −1 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ruthenium (III) bromide is a chemical compound of ruthenium and is one of the bromides . It is a black, crystalline solid.
Extraction and presentation
Ruthenium (III) bromide can be obtained directly from the elements at temperatures of 450 ° C and increased pressure.
An alternative is the reaction of ruthenium (VIII) oxide with hydrogen bromide .
properties
Ruthenium (III) bromide crystallizes in two different structures that merge at 383 K. Above this temperature the compound crystallizes in a hexagonal structure in the space group P 6 3 / mcm (space group no. 193) with the lattice parameters a = 652 pm and c = 589 pm as well as two formula units per unit cell .
Below 383 K, this structure is orthorhombically distorted and now occurs in the space group Pmmn (No. 59) with the lattice parameters a = 1126 pm, b = 587 pm and c = 650 pm as well as four formula units per unit cell. Long chains of face-sharing octahedra form in both structures; the distortion is caused by the formation of pairs of two ruthenium atoms.
Individual evidence
- ^ A b H. Hillebrecht, Th. Ludwig, G. Thiele: About Trihalides with TiI 3 Chain Structure . Proof of Pair Forming of Cations in -RuCl 3 and RuBr 3 by Temperature Dependent Single Crystal X-ray Analyzes. In: Journal of Inorganic and General Chemistry . tape 630 , 2004, pp. 2199-2204 , doi : 10.1002 / zaac.200400106 .
- ↑ a b c d e data sheet ruthenium (III) bromide, Ru 29% min from AlfaAesar, accessed on December 7, 2019 ( PDF )(JavaScript required) .
- ↑ ruthenium (III) bromide at webelements.com
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 2: Subgroup elements, lanthanoids, actinides, transactinides. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049590-4 , p. 1975 (reading sample: Part C - Subgroup elements. Google book search ).
- ↑ K. Brodersen, H.-K. Breitbach, G. Thiele: The fine structure of ruthenium (III) bromide . In: Journal of Inorganic and General Chemistry . tape 357 , no. 4-5 , 1967, pp. 162-171 , doi : 10.1002 / zaac.19683570402 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1671.

