Potassium metaborate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
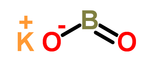
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium metaborate | |||||||||||||||
| other names |
Potassium borate |
|||||||||||||||
| Molecular formula | KBO 2 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 81.91 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.3 g cm −3 |
|||||||||||||||
| Melting point |
947 ° C |
|||||||||||||||
| boiling point |
1401 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium metaborate is an inorganic chemical compound of potassium from the group of borates .
Extraction and presentation
Potassium metaborate can be obtained by reacting potassium carbonate with boron trioxide or boron nitride .
properties
Potassium metaborate is a white solid. It has a crystal structure with the space group R 3 c (space group no. 167) . The connection exists as a trimer .
use
Potassium metaborate is used in fracking fluids .
Individual evidence
- ↑ a b c David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 0-8493-0595-0 , pp. 77 ( limited preview in Google Book search).
- ↑ a b c data sheet Potassium metaborate, ≥31% B 2 O 3 basis, ≥42% K 2 O basis from Sigma-Aldrich , accessed on February 21, 2019 ( PDF ).
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics . CRC Press, 2016, ISBN 978-1-4987-5429-3 , pp. 104 ( limited preview in Google Book search).
- ↑ R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 500 ( limited preview in Google Book Search).
- ^ RK Sharma: Chemistry Of Hydrides And Carbides . Discovery Publishing House, 2007, ISBN 978-81-8356-227-0 , pp. 314 ( limited preview in Google Book search).
- ↑ New Yearbook for Mineralogy, Geology and Paleontology: Treatises . E. Schweizerbart, 1940, OCLC 4669071 , p. 192 ( limited preview in Google Book search).
- ^ WH Zachariasen: The Crystal Structure of Potassium Metaborate, K3 (B3O6). In: The Journal of Chemical Physics. 5, 1937, p. 919, doi: 10.1063 / 1.1749962 .
- ↑ William A. Hart, OF Beumel, Thomas P. Whaley: The Chemistry of Lithium, Sodium, Potassium, Rubidium, Cesium and Francium Pergamon Texts in Inorganic Chemistry . Elsevier, 2013, ISBN 978-1-4831-8757-0 , pp. 449 ( limited preview in Google Book search).
- ^ Frank R. Spellman: Environmental Impacts of Hydraulic Fracturing . CRC Press, 2012, ISBN 978-1-4665-1467-6 , pp. 435 ( limited preview in Google Book search).


