Tungsten (IV) bromide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| __ W 4+ __ Br - | |||||||
| General | |||||||
| Surname | Tungsten (IV) bromide | ||||||
| other names |
Tungsten tetrabromide |
||||||
| Ratio formula | WBr 4 | ||||||
| Brief description |
black solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 503.456 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
240 ° C (sublimation) |
||||||
| solubility |
reacts with water |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Tungsten (IV) bromide is an inorganic chemical compound of the tungsten from the group of bromides .
Extraction and presentation
Tungsten (IV) bromide can be obtained by reacting tungsten (V) bromide with tungsten at above 350 ° C or with aluminum above 240 ° C.
properties
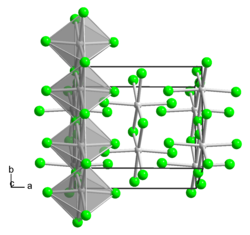
Tungsten (IV) bromide is in the form of diamagnetic black crystal needles. Its crystal structure is isotypic to that of niobium (IV) chloride (a = 849 pm, b = 929 pm, c = 725 pm).
Individual evidence
- ↑ a b c d Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1561.
- ↑ a b W. M. Haynes, David R. Lide, Thomas J. Bruno: CRC Handbook of Chemistry and Physics 2012-2013 . CRC Press, 2012, ISBN 1-4398-8049-2 , pp. 4–97 ( limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.

