Indium (I) bromide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ In + __ Br - | ||||||||||||||||
| Crystal system |
orthorhombic |
|||||||||||||||
| Space group |
Cmcm (No. 63) |
|||||||||||||||
| Lattice parameters |
|
|||||||||||||||
| General | ||||||||||||||||
| Surname | Indium (I) bromide | |||||||||||||||
| other names |
Indium monobromide |
|||||||||||||||
| Ratio formula | InBr | |||||||||||||||
| Brief description |
red-brown odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 194.73 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.96 g cm −3 |
|||||||||||||||
| Melting point |
220 ° C |
|||||||||||||||
| boiling point |
662 ° C |
|||||||||||||||
| solubility |
reacts with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−175 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Indium (I) bromide is an inorganic chemical compound of indium from the group of bromides .
Extraction and presentation
Indium (I) bromide can be obtained by reacting indium with bromine or indium (III) bromide in a vacuum at 300 to 400 ° C or with mercury (II) bromide at 350 ° C.
properties
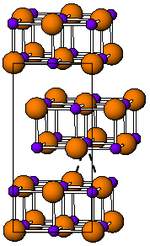
Indium (I) bromide is a red-brown, strongly irritating, odorless diamagnetic solid that reacts with water. The melt is red to black. The compound has an orthorhombic crystal structure in the space group Cmcm (space group no. 63) with the lattice parameters a = 446 pm, b = 1239 pm, c = 473 pm. The structure type is the thallium (I) iodide structure.
use
Indium (I) bromide is used, among other things, as a catalyst in organic chemistry and in sulfur lamps.
Individual evidence
- ↑ a b c d e f g h i data sheet Indium (I) bromide, Puratronic®, 99.999% (metals basis) from AlfaAesar, accessed on March 18, 2014 ( PDF )(JavaScript required) .
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 1393 (reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ).
- ↑ a b c Georg Brauer , with the assistance of Marianne Baudler a . a. (Ed.): Handbook of Preparative Inorganic Chemistry . 3rd, revised edition. tape I . Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 870 .
- ↑ Thomas Staffel, Gerd Meyer: The mono-, sesqui-, and dibromides of indium: InBr, In2Br3, and InBr2. In: Journal of Inorganic and General Chemistry. 552, 1987, pp. 113-122, doi : 10.1002 / zaac.19875520913 .
- ↑ Clovis Peppe, Rafael Pavodas Chagas: Indium (I) Bromide-Mediated Reductive Coupling of α, α-Dichloroketones to 1-Aryl-butane-1,4-diones. In: Synlett. 2004, pp. 1187-1190, doi : 10.1055 / s-2004-825591 .
- ^ A. Zissis: Light Sources 2004 Proceedings of the 10th International Symposium on the ... CRC Press, 2004, ISBN 0-7503-1007-3 , pp. 88 ( limited preview in Google Book search).



